(S)-(-)-Limonene Derivatives Containing (1H-1,2,3-Triazol-4-yl)methyl 4-Bromobenzoate
Abstract
:1. Introduction
2. Results and Discussions
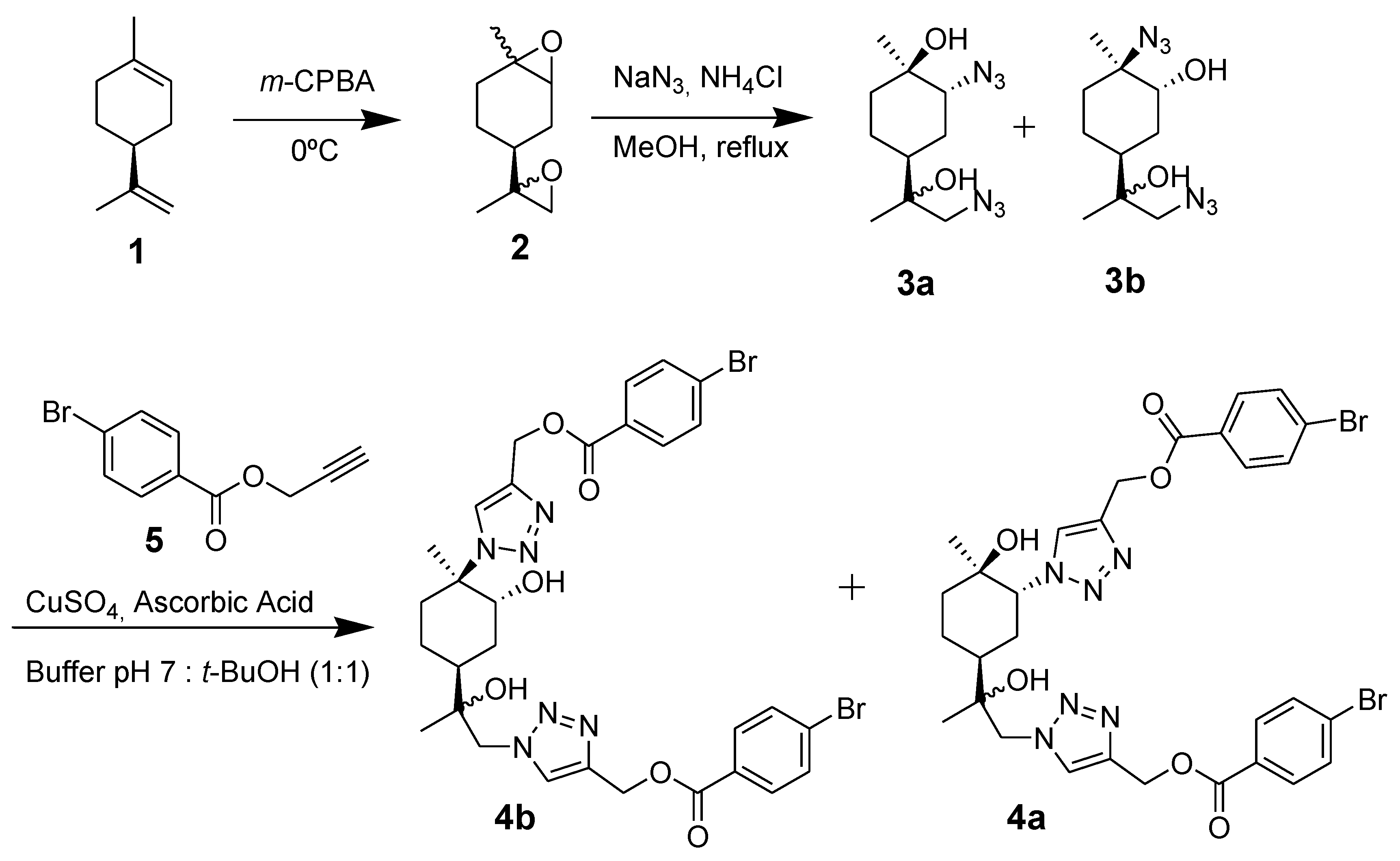
3. Experimental Section
(1-{2-[(1S,3R,4R)-3-(4-{[(4-bromophenyl)carbonyloxy]methyl}-1H-1,2,3-triazol-1-yl)-4-hydroxy-4-methylcyclohexyl]-2-hydroxypropyl}-1H-1,2,3-triazol-4-yl)methyl 4-bromobenzoate (4a) and {1-[(1R,2R,4S)-4-[1-(4-{[(4-bromophenyl)carbonyloxy]methyl}-1H-1,2,3-triazol-1-yl)-2-hydroxypropan-2-yl]-2-hydroxy-1-methylcyclohexyl]-1H-1,2,3-triazol-4-yl}methyl 4-bromobenzoate (4b)
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Supplementary File 5Supplementary File 6Supplementary File 7Acknowledgements
References
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Voronkov, M.V.; Gontcharov, A.V.; Kanamarlapudi, R.C.; Richardson, P.F.; Wang, Z.-M. Scalable Syntheses of Isomeric Limonene Aziridines from the Commercially Available Mixture of cis- and trans-Limonene Oxides. Org. Process Res. Dev. 2005, 9, 221–224. [Google Scholar] [CrossRef]
- Rostovstev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A Stepwise Huisgen Cycloaddition Process: Copper (I)- Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Valdomir, G.; Davyt, D. (S)-(-)-Limonene Derivatives Containing (1H-1,2,3-Triazol-4-yl)methyl 4-Bromobenzoate. Molbank 2011, 2011, M721. https://doi.org/10.3390/M721
Valdomir G, Davyt D. (S)-(-)-Limonene Derivatives Containing (1H-1,2,3-Triazol-4-yl)methyl 4-Bromobenzoate. Molbank. 2011; 2011(1):M721. https://doi.org/10.3390/M721
Chicago/Turabian StyleValdomir, Guillermo, and Danilo Davyt. 2011. "(S)-(-)-Limonene Derivatives Containing (1H-1,2,3-Triazol-4-yl)methyl 4-Bromobenzoate" Molbank 2011, no. 1: M721. https://doi.org/10.3390/M721



