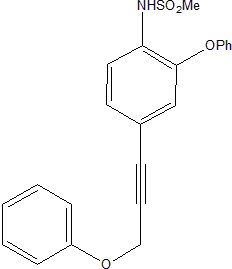
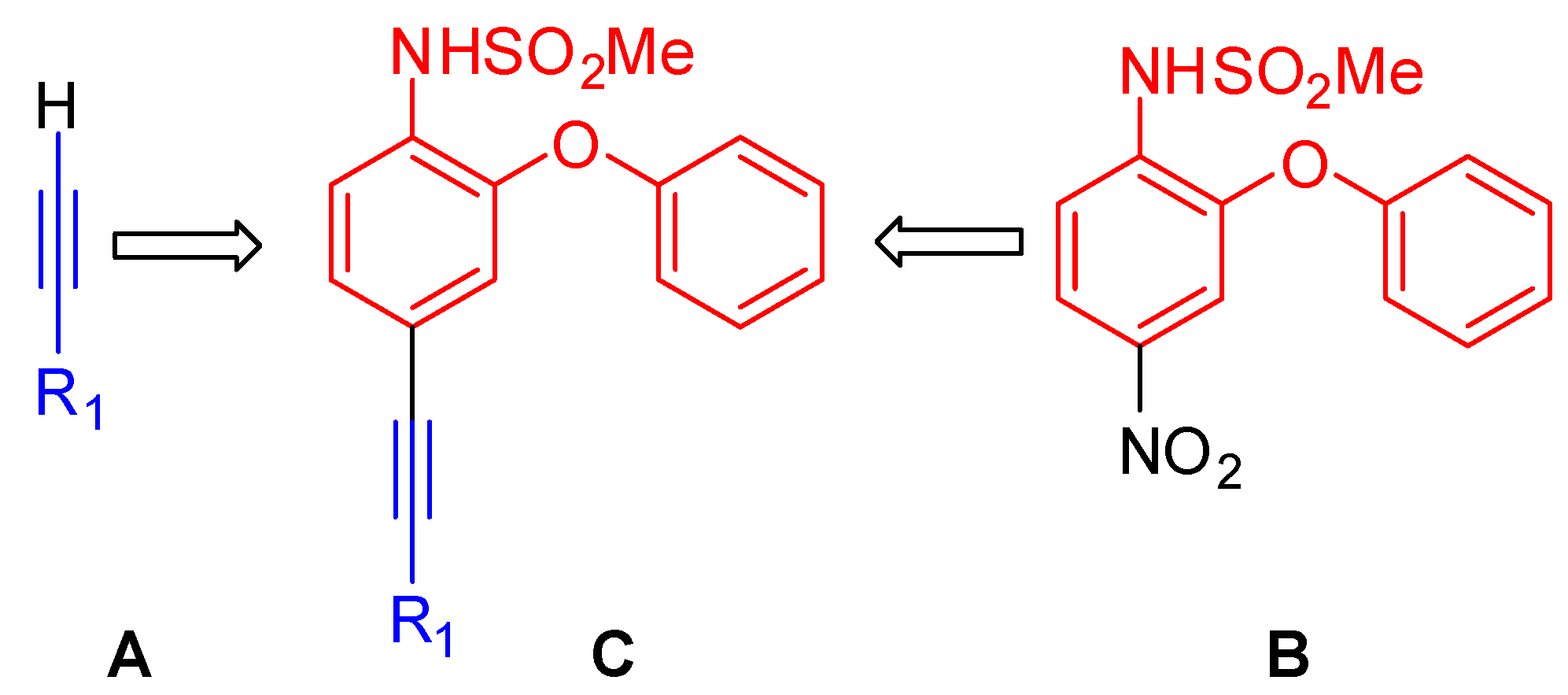
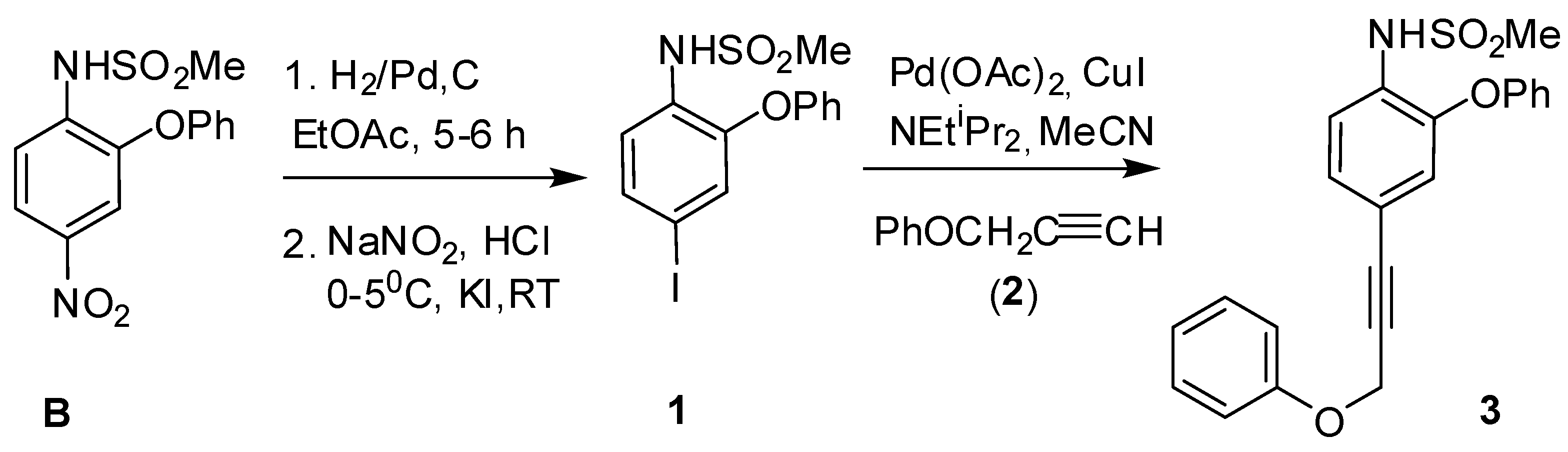
N-(2-Phenoxy-4-(3-phenoxyprop-1-ynyl)phenyl)methane Sulfonamide
Abstract
:Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgments
References
- Yu, L.; Wu, W.K.; Li, Z.J.; Liu, Q.C.; Li, H.T.; Wu, Y.C.; Cho, C.H. Enhancement of doxorubicin cytotoxicity on human esophageal squamous cell carcinoma cells by indomethacin and 4-[5-(4-chlorophenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]benzenesulfonamide (SC236) via inhibiting P-glycoprotein activity. Mol. Pharmacol. 2009, 75, 1364–1373. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.J.; Morrow, J.D. Analgesic-antipyretic and anti-inflammatory agents and drugs employed in the treatment of gout. In Goodman & Gilman’s The Pharmacological Basis of Therapeutics; Hardman, J.G., Limbird, L.E., Eds.; McGraw-Hill: New York, NY, USA, 2001; pp. 687–731. [Google Scholar]
- Pericherla, S.; Mareddy, J.; Rani, D.P.G.; Gollapudi, P.V.; Pal, S. Chemical modifications of nimesulide. J. Braz. Chem. Soc. 2007, 18, 384–390. [Google Scholar] [CrossRef]
- Reddy, L.V.; Nakka, M.; Suman, A.; Ghosh, S.; Helliwell, M.; Mukkanti, K.; Mukherjee, A.K.; Pal, S. Synthesis of novel quinoline analogues of nimesulide: An unusual observation. J. Heterocycl. Chem. 2011, 48, 555–562. [Google Scholar] [CrossRef]
- Durgadas, S.; Chatare, V.K.; Mukkanti, K.; Pal, S. Palladium-mediated synthesis of novel nimesulide derivatives. Appl. Organometal. Chem. 2010, 24, 680–684. [Google Scholar] [CrossRef]
- Kankanala, K.; Reddy, V.R.; Mukkanti, K.; Pal, S. Lewis Acid Free High Speed Synthesis of Nimesulide-Based Novel N-Substituted Cyclic Imides. J. Braz. Chem. Soc. 2010, 21, 1060–1064. [Google Scholar] [CrossRef]
- Reddy, L.V.; Kethavath, M.; Nakka, M.; Beevi, S.S.; Mangamoori, L.N.; Mukkanti, K.; Pal, S. Design and synthesis of novel cytotoxic agents based on combined framework of quinoline and nimesulide. J. Heterocycl. Chem. 2012, 49, 80–87. [Google Scholar] [CrossRef]
- Pal, S.; Durgadas, S.; Nallapati, S.B.; Mukkanti, K.; Kapavarapu, R.; Meda, C.L.T.; Parsa, K.V.L.; Pal, M. Novel 1-alkynyl substituted 1,2-dihydroquinoline derivatives from nimesulide (and their 2-oxo analogues): A new strategy to identify inhibitors of PDE4B. Bioorg. Med. Chem. Lett. 2011, 21, 6573–6576. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Ghosh, S.; Kavitha, K.; Reddy, V.R.; Mukkanti, K.; Pal, S.; Mukherjee, A.K. Crystal structure and electronic properties of two nimesulide derivatives: A combined X-ray powder diffraction and quantum mechanical study. Chem. Phys. Lett. 2010, 493, 151–157. [Google Scholar] [CrossRef]
- Kavitha, K.; Varsha, P.; Mukkanti, K.; Reddy, V.R.; Pal, S. N-(4-Methylsulfonamido-3-phenoxyphenyl)-9,10-dihydro-9,10-ethanoanthracene-11,12-dicarboximide. Molbank 2011, 2011, M740. [Google Scholar]
- Sonogashira, K.; Tohda, Y.; Hagihara, N. A convenient synthesis of acetylenes: Catalytic substitutions of acetylenic hydrogen with bromoalkenes, iodoarenes and bromopyridines. Tetrahedron Lett. 1975, 16, 4467–4470. [Google Scholar] [CrossRef]
- Chinchilla, R.; Najera, C. The Sonogashira Reaction: A Booming Methodology in Synthetic Organic Chemistry. Chem. Rev. 2007, 107, 874–922. [Google Scholar] [CrossRef] [PubMed]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Durgadas, S.; Mukkanti, K.; Pal, S. N-(2-Phenoxy-4-(3-phenoxyprop-1-ynyl)phenyl)methane Sulfonamide. Molbank 2012, 2012, M751. https://doi.org/10.3390/M751
Durgadas S, Mukkanti K, Pal S. N-(2-Phenoxy-4-(3-phenoxyprop-1-ynyl)phenyl)methane Sulfonamide. Molbank. 2012; 2012(1):M751. https://doi.org/10.3390/M751
Chicago/Turabian StyleDurgadas, Shylaprasad, Khagga Mukkanti, and Sarbani Pal. 2012. "N-(2-Phenoxy-4-(3-phenoxyprop-1-ynyl)phenyl)methane Sulfonamide" Molbank 2012, no. 1: M751. https://doi.org/10.3390/M751
APA StyleDurgadas, S., Mukkanti, K., & Pal, S. (2012). N-(2-Phenoxy-4-(3-phenoxyprop-1-ynyl)phenyl)methane Sulfonamide. Molbank, 2012(1), M751. https://doi.org/10.3390/M751