tert-Butyl 1-(Furan-2-yl)-4-oxo-2,3,7-triazaspiro[4.5]dec-1-ene-7-carboxylate
Abstract
:Introduction
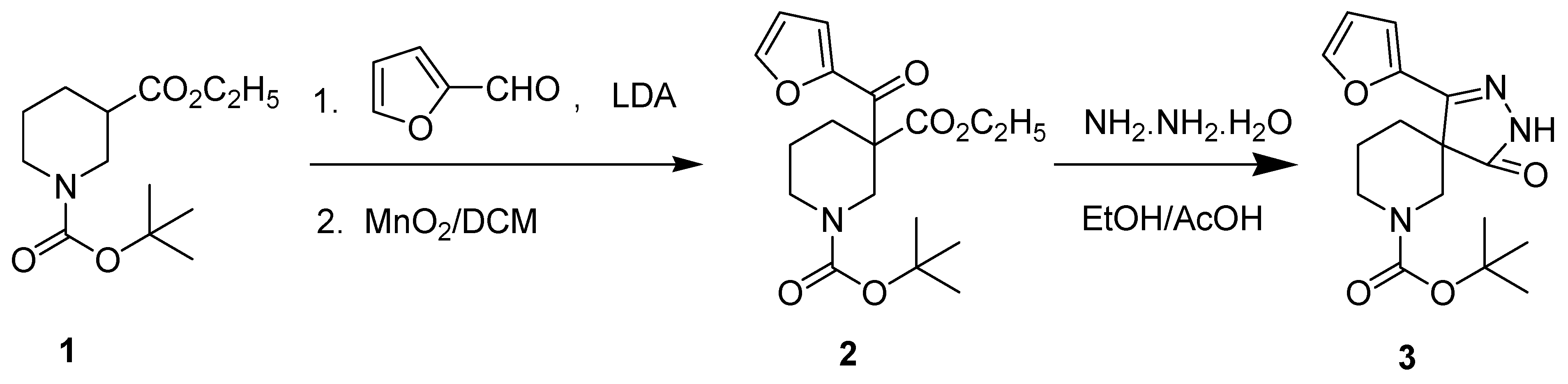
Results and Discussion
Experimental
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgments
References
- Mead, K.T.; Brewer, B.N. Strategies in spiroketal synthesis revisited: Recent applications and advances. Curr. Org. Chem. 2003, 7, 227–256. [Google Scholar] [CrossRef]
- Shi, Z.-J.; Zhang, S.-L.; Cao, W.-G.; Deng, H.-M. Facile synthesis of a series of perfluoroalkyl-containing tetra-spirocyclic compounds and their spectral analysis. Chin. J. Chem. 2008, 26, 2103–2106. [Google Scholar] [CrossRef]
- Sannigrahi, M. Stereocontrolled synthesis of spirocyclics. Tetrahedron 1999, 55, 9007–9071. [Google Scholar] [CrossRef]
- Zhang, Z.-H. Synthesis and application of chiral spiro ligands in asymmetric catalysis. Chin. J. Org. Chem. 2005, 25, 355–363. [Google Scholar]
- Arai, M.A.; Kuraishi, M.; Arai, T.; Sasai, H. A new asymmetric Wacker-type cyclization and tandem cyclization promoted by Pd(II)-spiro bis(isoxazoline) catalyst. J. Am. Chem. Soc. 2001, 123, 2907–2908. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.-Y.; Wang, Y.; William, J.B. The investigation of polymerization mechanism of 7-methylene-2-methyl-1,4,6-triox-aspiro(4,4)nonane. Acta Polym. Sin. 1989, 1, 18–24. [Google Scholar]
- Aslanian, R.G; Bennett, C.E.; Burnett, D.A.; Chan, T.-Y.; Kiselgof, E.Y.; Knutson, C.E.; Harris, J.M.; Mc Kittrick, B.A.; Palani, A.; Smith, E.M.; Vaccaro, H.M.; Xiao, D.; Kim, H.M. Azetidinone derivatives and methods of use thereof. WO2008033464(A2), 20 March 2008. [Google Scholar]
- Theodoridis, G.; Rosen, D.; Zhang, S.; Yeager, W.H.; Henrie, I.R.N.; Ahmed, S.Z.; Men, H.; Donovan, S.F. N-Substituted azacycles. WO2005036961(A2), 28 April 2005. [Google Scholar]
- Corey, E.J.; Gilman, N.W.; Ganem, B.E. New methods for the oxidation of aldehydes to carboxylic acids and esters. J. Am. Chem. Soc. 1968, 90, 5616–5617. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Srinivasan, R.; Narayana, B.; Samshuddin, S.; Sarojini, B.K. tert-Butyl 1-(Furan-2-yl)-4-oxo-2,3,7-triazaspiro[4.5]dec-1-ene-7-carboxylate. Molbank 2012, 2012, M757. https://doi.org/10.3390/M757
Srinivasan R, Narayana B, Samshuddin S, Sarojini BK. tert-Butyl 1-(Furan-2-yl)-4-oxo-2,3,7-triazaspiro[4.5]dec-1-ene-7-carboxylate. Molbank. 2012; 2012(2):M757. https://doi.org/10.3390/M757
Chicago/Turabian StyleSrinivasan, Rajagopalan, Badiadka Narayana, Seranthimata Samshuddin, and Balladka Kunhanna Sarojini. 2012. "tert-Butyl 1-(Furan-2-yl)-4-oxo-2,3,7-triazaspiro[4.5]dec-1-ene-7-carboxylate" Molbank 2012, no. 2: M757. https://doi.org/10.3390/M757




