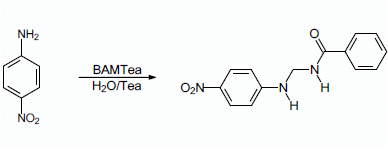
N-{[(4-Nitrophenyl)amino]methyl}benzamide
Abstract
:Introduction
Results and Discussion
Experimental
Materials
Instrumentation
Synthesis of N-{[(4-nitrophenyl)amino]methyl}benzamide
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgments
Conflicts of Interest
References
- Popovski, E. Synthesis of N-(N'-benzoylhydrazinomethyl)benzamide. Molbank 2007, M525. [Google Scholar] [CrossRef]
- Popovski, E. Synthesis of N-[N'-(2-hydroxy-2,2-diphenylacethyl)hydrazinomethyl]benzamide. Molbank 2007, M526. [Google Scholar] [CrossRef]
- Popovski, E.; Klisarova, L.; Vikic-Topic, D. Benzamidomethylation with (benzamidomethyl)triethylammonium chloride. 2. A simple method for benzamidomethylation of thiols, amines and carboxylic acids. Molecules 2000, 5, 927–936. [Google Scholar] [CrossRef]
- Schioppacassi, G.; Morvillo, E.; Della Bruna, C.; Franceschi, G.; Foglio, M. In vitro and in vivo evaluation of benzamidomethyl-benzylpenicillinate (fi7303). A new 'repository' form. Chemotherapy 1978, 24, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Bundgaard, H.; Nielsen, N.M.; Buur, A. Aspirin prodrugs: Synthesis and hydrolysis of 2-acetoxybenzoate esters of various N-(hydroxyalkyl) amides. Int. J. Pharm. 1988, 44, 151–158. [Google Scholar] [CrossRef]
- Moreira, R.; Calheiros, T.; Cabrita, J.; Mendes, E.; Pimentel, M.; Iley, J. Acyloxymethyl as a drug protecting group. Part 3. Tertiary O-amidomethyl esters of penicillin g: Chemical hydrolysis and anti-bacterial activity. Pharm. Res. 1996, 13, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Böhme, H.; Raude, E. Zur acylspaltung N-acylierter aminale. Chem. Ber. 1981, 114, 3421–3429. [Google Scholar] [CrossRef]
- Unterhalt, B.; Mohr, R.; Thamer, D. Nitramine, 17. Mitt. Alkyl-arylmethyl-nitramine. Arch. Pharm. 1985, 318, 878–882. [Google Scholar] [CrossRef]
- Schönenberger, H.; Petter, A.; Kuehling, V.; Bindl, L. Synthesis and testing the cardiocirculatory effects of N-(3'-methoxybenzamidomethyl)-D-norephedrine and analogous compounds. Arch. Pharm. 1976, 309, 289–301. [Google Scholar] [CrossRef]
- Haworth, R.D.; Peacock, D.H.; Smith, W.R.; MacGillivray, R. 569. The action of formaldehyde on proteins. Part II. Some reactions of N-hydroxymethylamides. J. Chem. Soc. 1952, 2972–2980. [Google Scholar] [CrossRef]
- Lazarevic, M.D.; Csanadi, J.; Klisarova, L. Synthesis of new benzotriazole derivatives. Bull. Chem. Technol. Macedonia 1995, 14, 19–22. [Google Scholar]
- Watase, Y.; Terao, Y.; Sekiya, M. Synthesis of N-(alkylaminomethyl) amides. Chem. Pharm. Bull. 1973, 21, 2775–2778. [Google Scholar] [CrossRef]
- Zanatta, N.; Rittner, R. Synthesis of 4-substituted N-[(dimethylamino)methyl]benzamides: New compounds. J. Pharm. Sci. 1983, 72, 831–832. [Google Scholar] [CrossRef] [PubMed]
- Zanatta, N.; Rittner, R. 13C NMR of 4-substituted N-[(dimethylamino)methyl]benzamides. Spectrosc. Lett. 1987, 20, 577–582. [Google Scholar] [CrossRef]

© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Buzarevski, A.; Mikhova, B.; Popovski, E. N-{[(4-Nitrophenyl)amino]methyl}benzamide. Molbank 2014, 2014, M821. https://doi.org/10.3390/M821
Buzarevski A, Mikhova B, Popovski E. N-{[(4-Nitrophenyl)amino]methyl}benzamide. Molbank. 2014; 2014(1):M821. https://doi.org/10.3390/M821
Chicago/Turabian StyleBuzarevski, Antonio, Bozhana Mikhova, and Emil Popovski. 2014. "N-{[(4-Nitrophenyl)amino]methyl}benzamide" Molbank 2014, no. 1: M821. https://doi.org/10.3390/M821