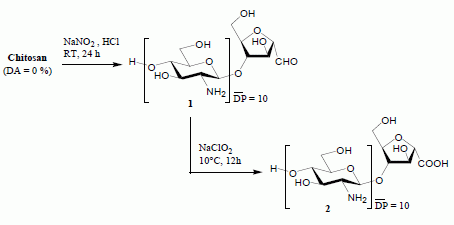
Chitooligosaccharide-2,5-anhydro-D-mannonic Acid
Abstract
:Results and Discussion
Experimental
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Dash, M.; Chiellini, F.; Ottenbriteb, R.M.; Chiellini, E. Chitosan: A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Yeul, V.S.; Rayalu, S.S. Unprecedented Chitin and Chitosan: A Chemical Overview. J. Polym. Environ. 2013, 21, 606–614. [Google Scholar] [CrossRef]
- Aam, B.B.; Heggset, E.B.; Norberg, A.L.; Sørlie, M.; Vårum, K.M.; Eijsink, V.G.H. Production of chitooligosaccharides and their potential applications in medicine. Mar. Drugs 2010, 8, 1482–1517. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Liu, P.; Zhang, J.; Chen, J. Biological activities of chitosan and chitooligosaccharides. Food Hydrocolloids 2011, 25, 170–179. [Google Scholar] [CrossRef]
- Lodhi, G.; Kim, Y.-S.; Hwang, J.-W.; Kim, S.-K.; Jeon, Y.-J.; Je, J.-Y.; Ahn, C.-B.; Moon, S.-H.; Jeon, B.-T.; Park, P.-J. Chitooligosaccharide and Its Derivatives: Preparation and Biological Applications. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Trombotto, S.; Ladavière, C.; Delolme, F.; Domard, A. Chemical preparation and structural characterization of a homogeneous series of chitin/chitosan oligomers. Biomacromolecules 2008, 9, 1731–1738. [Google Scholar] [CrossRef] [PubMed]
- Tommeraas, K.; Varum, K.M.; Christensen, B.E.; Smidsrod, O. Preparation and characterization of oligosaccharides produced by nitrous acid depolymerization of chitosans. Carbohydr. Res. 2001, 333, 137–144. [Google Scholar] [CrossRef]
- Hussain, I.; Singh, T.; Chittenden, C. Preparation of chitosan oligomers and characterization: their antifungal activities and decay resistance. Holzforschung 2012, 66, 119–125. [Google Scholar] [CrossRef]
- Mourya, V.K.; Inamdar, N.N.; Choudhari, Y.M. Chitooligosaccharides: Synthesis, characterization and applications. Polym. Sci. Ser. A Polym. Phys. 2011, 53, 583–612. [Google Scholar] [CrossRef]
- Sashiwa, H.; Saimoto, H.; Shigemas, Y.; Tokura, S. Chitooligosaccharides: Synthesis, characterizations and applications. Carbohydr. Res. 1993, 242, 167–172. [Google Scholar] [CrossRef]
- Lin, C.-W.; Lin, J.-C. Characterization and blood coagulation evaluation of the water-soluble chitooligosaccharides prepared by a facile fractionation method. Biomacromolecules 2003, 4, 1691–1697. [Google Scholar] [CrossRef] [PubMed]
- Salim, E.; Galais, A.; Trombotto, S. 4-(Hexyloxy)aniline-linked chitooligosaccharide-2,5-anhydro-d-mannofuranose. Molbank 2014, 2014, M815. [Google Scholar] [CrossRef]
- Note that in the 1H-NMR analysis conditions used here, the aldehyde group of the amf-units does not exist in its free (-CHO) form but exclusively in its hydrated (–CH(OH)2) form.
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Salim, E.; Ailincai, D.; Trombotto, S. Chitooligosaccharide-2,5-anhydro-D-mannonic Acid. Molbank 2014, 2014, M832. https://doi.org/10.3390/M832
Salim E, Ailincai D, Trombotto S. Chitooligosaccharide-2,5-anhydro-D-mannonic Acid. Molbank. 2014; 2014(3):M832. https://doi.org/10.3390/M832
Chicago/Turabian StyleSalim, Emil, Daniela Ailincai, and Stéphane Trombotto. 2014. "Chitooligosaccharide-2,5-anhydro-D-mannonic Acid" Molbank 2014, no. 3: M832. https://doi.org/10.3390/M832