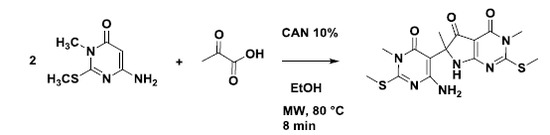
6-(4-Amino-1-methyl-2-(methylthio)-6-oxo-1,6-dihydro-pyrimidin-5-yl)-3,6-dimethyl-2-(methylthio)-6,7-dihydro-3H-pyrrolo[2,3-d]pyrimidine-4,5-dione
Abstract
:Introduction
Results and Discussion
Experimental
General Information
Procedure for the Synthesis of 6-(4-Amino-1-methyl-2-(methylthio)-6-oxo-1,6-dihydropyrimidin-5-yl)-3,6-dimethyl-2-(methylthio)-6,7-dihydro-3H-pyrrolo[2,3-d]pyrimidine-4,5-dione
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgments
Author Contributions
Conflicts of Interest
References
- Choudhury, A.; Chen, H.; Nilsen, C.N.; Sorgi, K.L. A chemoselective aniline–chloropyrimidine coupling in a competing electrophilic environment. Tetrahedron Lett. 2008, 49, 102–105. [Google Scholar] [CrossRef]
- Brændvang, M.; Gundersen, L.-L. Efficient and regioselective N-1 alkylation of 4-chloropyrazolo[3,4-d]pyrimidine. Tetrahedron Lett. 2007, 48, 3057–3059. [Google Scholar] [CrossRef]
- Peng, Z.; Journet, M.; Humphrey, G. A Highly Regioselective Amination of 6-Aryl-2,4-dichloropyrimidine. Org. Lett. 2006, 8, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Girreser, U.; Heber, D.; Schütt, M. Synthesis of 6-substituted 7-aryl-5,6-dihydropyrido[2,3-d]pyrimidine(1H,3H)-2,4-diones using the Vilsmeier reaction. Tetrahedron 2004, 60, 11511–11517. [Google Scholar] [CrossRef]
- Boudet, N.; Knochel, P. Chemo- and regioselective functionalization of uracil derivatives. Applications to the synthesis of oxypurinol and emivirine. Org. Lett. 2006, 8, 3737–40. [Google Scholar] [CrossRef] [PubMed]
- Baraldi, P.; Bovero, A.; Fruttarolo, F.; Romagnoli, R.; Aghzadeh, M.; Preti, D.; Varani, K.; Borea, P.; Moorman, A. New strategies for the synthesis of A3 adenosine receptor antagonists. Bioorg. Med. Chem. 2003, 11, 4161–4169. [Google Scholar] [CrossRef]
- Baraldi, P.G.; Cacciari, B.; Romagnoli, R.; Spalluto, G.; Moro, S.; Klotz, K.; Leung, E.; Varani, K.; Gessi, S.; Merighi, S.; et al. Selective Human A3 Adenosine Receptor Antagonists: Influence of the Chain at the N8 Pyrazole Nitrogen. J. Med. Chem. 2000, 43, 4768–4780. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, N.S.; El-Bendary, E.R.; El-Ashry, S.M.; El-Kerdawy, M.M. Synthesis and antitumor activity of new sulfonamide derivatives of thiadiazolo[3,2-a]pyrimidines. Eur. J. Med. Chem. 2011, 46, 3714–3720. [Google Scholar] [CrossRef] [PubMed]
- Fares, M.; Abou-Seri, S.M.; Abdel-Aziz, H.; Abbas, S.; Youssef, M.M.; Eladwy, R.A. Synthesis and antitumor activity of pyrido [2,3-d]pyrimidine and pyrido[2,3-d][1,2,4]triazolo[4,3-a]pyrimidine derivatives that induce apoptosis through G1 cell-cycle arrest. Eur. J. Med. Chem. 2014, 83, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Al-Omary, F.A.M.; Hassan, G.S.; El-Messery, S.M.; El-Subbagh, H.I. Substituted thiazoles V. synthesis and antitumor activity of novel thiazolo[2,3-b]quinazoline and pyrido[4,3-d]thiazolo[3,2-a]pyrimidine analogues. Eur. J. Med. Chem. 2012, 47, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wu, S.; Wang, Y.; Li, R.; Wang, J.; Wang, L.; Zhao, Y.; Gong, P. Design, synthesis and biological evaluation of novel thieno[3,2-d]pyrimidine derivatives possessing diaryl semicarbazone scaffolds as potent antitumor agents. Eur. J. Med. Chem. 2014, 87, 782–93. [Google Scholar] [CrossRef] [PubMed]
- Hilmy, K.M.H.; Khalifa, M.M.A.; Hawata, M.A.A.; Keshk, R.M.A.; El-Torgman, A.A. Synthesis of new pyrrolo[2,3-d]pyrimidine derivatives as antibacterial and antifungal agents. Eur. J. Med. Chem. 2010, 45, 5243–5250. [Google Scholar] [CrossRef] [PubMed]
- Gholap, A.R.; Toti, K.S.; Shirazi, F.; Deshpande, M.V.; Srinivasan, K.V. Efficient synthesis of antifungal pyrimidines via palladium catalyzed Suzuki/Sonogashira cross-coupling reaction from Biginelli 3,4-dihydropyrimidin-2(1H)-ones. Tetrahedron 2008, 64, 10214–10223. [Google Scholar] [CrossRef]
- Bhalgat, C.M.; Ramesh, B. Synthesis, antimicrobial screening and structure–activity relationship of novel pyrimidines and their thioethers. Bull. Fac. Pharmacy, Cairo Univ. 2014, 52, 259–267. [Google Scholar] [CrossRef]
- Saikia, L.; Das, B.; Bharali, P.; Thakur, A.J. A convenient synthesis of novel 5-aryl-pyrido[2,3-d]pyrimidines and screening of their preliminary antibacterial properties. Tetrahedron Lett. 2014, 55, 1796–1801. [Google Scholar] [CrossRef]
- Al-Adiwish, W.M.; Tahir, M.I.M.; Siti-Noor-Adnalizawati, A.; Hashim, S.F.; Ibrahim, N.; Yaacob, W. Synthesis, antibacterial activity and cytotoxicity of new fused pyrazolo[1,5-a]pyrimidine and pyrazolo[5,1-c][1,2,4]triazine derivatives from new 5-aminopyrazoles. Eur. J. Med. Chem. 2013, 64, 464–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallis, M.P.; Mahmood, N.; Fraser, W. Synthesis and anti-HIV activity of C4-modified pyrimidine nucleosides. Il Farmaco 1999, 54, 83–89. [Google Scholar] [CrossRef]
- Gazivoda, T.; Raić-Malić, S.; Kristafor, V.; Makuc, D.; Plavec, J.; Bratulić, S.; Kraljević-Pavelić, S.; Pavelić, K.; Naesens, L.; Andrei, G.; et al. Synthesis, cytostatic and anti-HIV evaluations of the new unsaturated acyclic C-5 pyrimidine nucleoside analogues. Bioorg. Med. Chem. 2008, 16, 5624–5634. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Du, D.; Rai, D.; Wang, L.; Liu, H.; Zhan, P.; de Clercq, E.; Pannecouque, C.; Liu, X. Fused heterocyclic compounds bearing bridgehead nitrogen as potent HIV-1 NNRTIs. Part 1: design, synthesis and biological evaluation of novel 5,7-disubstituted pyrazolo[1,5-a]pyrimidine derivatives. Bioorg. Med. Chem. 2014, 22, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Gangjee, A.; Namjoshi, O.; Yu, J.; Ihnat, M.; Thorpe, J.E.; Bailey-Downs, L.C. N2-Trimethylacetyl substituted and unsubstituted-N4-phenylsubstituted-6-(2-pyridin-2-ylethyl)-7H-pyrrolo[2,3-d]pyrimidine-2,4-diamines: Design, cellular receptor tyrosine kinase inhibitory activities and in vivo evaluation as antiangiogenic, antimetastatic and antitumor agents. Bioorg. Med. Chem. 2013, 21, 1312–1323. [Google Scholar] [PubMed]
- Gangjee, A.; Zaware, N.; Raghavan, S.; Yang, J.; Thorpe, J.E.; Ihnat, M.A. N4-(3-Bromophenyl)-7-(substituted benzyl) pyrrolo[2,3-d]pyrimidines as potent multiple receptor tyrosine kinase inhibitors: Design, synthesis, and in vivo evaluation. Bioorg. Med. Chem. 2012, 20, 2444–2454. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, S.D.; Wilson, J.W.; Deanda, F.; Patnaik, S.; Redman, A.M.; Yang, B.; Shewchuk, L.; Sabbatini, P.; Leesnitzer, M.A.; Groy, A.; et al. Discovery of 4,6-bis-anilino-1H-pyrrolo[2,3-d]pyrimidines: Potent inhibitors of the IGF-1R receptor tyrosine kinase. Bioorg. Med. Chem. Lett. 2009, 19, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Kaspersen, S.J.; Sørum, C.; Willassen, V.; Fuglseth, E.; Kjøbli, E.; Bjørkøy, G.; Sundby, E.; Hoff, B.H. Synthesis and in vitro EGFR (ErbB1) tyrosine kinase inhibitory activity of 4-N-substituted 6-aryl-7H-pyrrolo[2,3-d]pyrimidine-4-amines. Eur. J. Med. Chem. 2011, 46, 6002–6014. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, Y.; Tao, L.; Wang, Q.; Wang, S.; Hu, W.; Pan, Z.; Yang, Q.; Cui, Y.; Ge, Z.; et al. Synthesis and anti-HIV-1 activity of 4-substituted-7-(2'-deoxy-2'-fluoro-4'-azido-β-d-ribofuranosyl)pyrrolo[2,3-d]pyrimidine analogues. Bioorg. Med. Chem. Lett. 2011, 21, 6770–6772. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.P.; Frey, K.M.; Wang, Y.; Jain, H.K.; Gangjee, A.; Anderson, K.S. Substituted pyrrolo[2,3-d]pyrimidines as Cryptosporidium hominis thymidylate synthase inhibitors. Bioorg. Med. Chem. Lett. 2013, 23, 5426–5428. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, J.; Acosta, P.A.; Cruz, S.; Abonía, R.; Insuasty, B.; Nogueras, M.; Cobo, J. Generation of pyrrolo[2,3-d]pyrimidines. Unexpected products in the multicomponent reaction of 6-aminopyrimidines, dimedone, and arylglyoxal. Tetrahedron Lett. 2010, 51, 5443–5447. [Google Scholar] [CrossRef]
- Barnett, C.J.; Grubb, L.M. Synthesis of pyrrolo[2,3-d]pyrimidines via cyclocondensation of β-alkoxy and β-amino-α-bromoaldehydes. Tetrahedron Lett. 2000, 41, 9741–9745. [Google Scholar] [CrossRef]
- Bundy, G.L.; Ayer, D.E.; Banitt, L.S.; Belonga, K.L.; Mizsak, S.A.; Palmer, J.R.; Tustin, J.M.; Chin, J.E.; Hall, E.D.; Linseman, K.L.; et al. Synthesis of Novel 2,4-Diaminopyrrolo-[2,3-d]pyrimidines with Antioxidant, Neuroprotective, and Antiasthma Activity. J. Med. Chem. 1995, 38, 4161–4163. [Google Scholar] [CrossRef] [PubMed]
- Lipton, M.F.; Mauragis, M.A.; Veley, M.F.; Bundy, G.L.; Banitt, L.S.; Dobrowolski, P.J.; Palmer, J.R.; Schwartz, T.M.; Zimmerman, D.C. Four Generations of Pyrrolopyrimidines. In From Bench to Pilot Plant; Nafissi, M., Ragan, J.A., DeVries, K.M., Eds.; American Chemical Society: Washington, DC, USA, 2002; pp. 101–112. [Google Scholar]
- Gálvez, J.; Quiroga, J.; Insuasty, B.; Abonia, R. Microwave-assisted and iodine mediated synthesis of 5-n-alkyl-cycloalkane[d]-pyrazolo[3,4-b]pyridines from 5-aminopyrazoles and cyclic ketones. Tetrahedron Lett. 2014, 55, 1998–2002. [Google Scholar] [CrossRef]
- Quiroga, J.; Diaz, Y.; Bueno, J.; Insuasty, B.; Abonia, R.; Ortiz, A.; Nogueras, M.; Cobo, J. Microwave induced three-component synthesis and antimycobacterial activity of benzopyrazolo[3,4-b]quinolindiones. Eur. J. Med. Chem. 2014, 74, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Insuasty, B.; Becerra, D.; Quiroga, J.; Abonia, R.; Nogueras, M.; Cobo, J. Synthesis of Novel Pyrimido[4,5-b]quinolin-4-ones with Potential Antitumor Activity. J. Heterocycl. Chem. 2013, 50, 506–512. [Google Scholar] [CrossRef]
- Insuasty, B.; Becerra, D.; Quiroga, J.; Abonia, R.; Nogueras, M.; Cobo, J. Microwave-assisted synthesis of pyrimido[4,5-b][1,6]naphthyridin-4(3H)-ones with potential antitumor activity. Eur. J. Med. Chem. 2013, 60, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, J.; Portillo, S.; Pérez, A.; Gálvez, J.; Abonia, R.; Insuasty, B. An efficient synthesis of pyrazolo[3,4-b]pyridine-4-spiroindolinones by a three-component reaction of 5-aminopyrazoles, isatin, and cyclic β-diketones. Tetrahedron Lett. 2011, 52, 2664–2666. [Google Scholar] [CrossRef]
- Quiroga, J.; Trilleras, J.; Pantoja, D.; Abonía, R.; Insuasty, B.; Nogueras, M.; Cobo, J. Microwave-assisted synthesis of pyrazolo[3,4-b]pyridine-spirocycloalkanediones by three-component reaction of 5-aminopyrazole derivatives, paraformaldehyde and cyclic β-diketones. Tetrahedron Lett. 2010, 51, 4717–4719. [Google Scholar] [CrossRef]
- Quiroga, J.; Trilleras, J.; Insuasty, B.; Abonía, R.; Nogueras, M.; Marchal, A.; Cobo, J. A straightforward synthesis of pyrimido[4,5-b]quinoline derivatives assisted by microwave irradiation. Tetrahedron Lett. 2010, 51, 1107–1109. [Google Scholar] [CrossRef]
- Sridharan, V.; Mene, J.C. Cerium (IV) Ammonium Nitrate as a Catalyst in Organic Synthesis. Chem. Rev. 2010, 110, 3805–3849. [Google Scholar] [CrossRef] [PubMed]


© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romo, P.E.; Quiroga, J.; Insuasty, B.; Nogueras, M.; Cobo, J. 6-(4-Amino-1-methyl-2-(methylthio)-6-oxo-1,6-dihydro-pyrimidin-5-yl)-3,6-dimethyl-2-(methylthio)-6,7-dihydro-3H-pyrrolo[2,3-d]pyrimidine-4,5-dione. Molbank 2015, 2015, M842. https://doi.org/10.3390/M842
Romo PE, Quiroga J, Insuasty B, Nogueras M, Cobo J. 6-(4-Amino-1-methyl-2-(methylthio)-6-oxo-1,6-dihydro-pyrimidin-5-yl)-3,6-dimethyl-2-(methylthio)-6,7-dihydro-3H-pyrrolo[2,3-d]pyrimidine-4,5-dione. Molbank. 2015; 2015(1):M842. https://doi.org/10.3390/M842
Chicago/Turabian StyleRomo, Pablo E., Jairo Quiroga, Braulio Insuasty, Manuel Nogueras, and Justo Cobo. 2015. "6-(4-Amino-1-methyl-2-(methylthio)-6-oxo-1,6-dihydro-pyrimidin-5-yl)-3,6-dimethyl-2-(methylthio)-6,7-dihydro-3H-pyrrolo[2,3-d]pyrimidine-4,5-dione" Molbank 2015, no. 1: M842. https://doi.org/10.3390/M842