Abstract
5-Methyl-3,8-di-(2-amino-4-bromophenyl)-4,9-dioxa-1,2,6,7-tetraaza-5λ5-phosphaspiro[4.4]nona-2,7-diene was obtained by condensation of 2-amino-5-bromobenzohydrazide and methylphosphonyl dichloride in the presence of triethylamine. An initial biological screening was performed for the resulting product. The synthesized compound showed relatively strong cytotoxic activity, which was, however, similar for cancer and non-cancer cell lines.
1. Introduction
After the development of cyclophosphamide 1 (Figure 1)—a potent antineoplastic agent [1]—medicinal chemists focused their attention on the application of phosphorus heterocycles as potential antiproliferative agents. Although no other phosphoheterocycle repeated the tremendous success of Cyclophosphamide 1, some of them exhibited noticeable cytotoxic and antiproliferative effects. Bull reported [2] that some of the isoquino[2,1-c][1,3,2]benzodiazaphosphorine derivatives 2a–c exhibited promising anticancer effects against Ehrlich ascites carcinoma and P-388 lymphocytic leukemia cells. Hudson observed [3] that a dimer of 3-methyl-1(2,4,6-triisopropylphenyl)phosphole oxide 3 showed GI50 values in the micromolar range against two leukemia cell lines (RPMI-8226 and SR), non-small cell lung cancer (NCI-H460), colon cancer (COLO 205), and melanoma (SK-Mel-5 and UACC-62). In the same article, he also observed a moderate antiproliferative effect of phosphinine 1-oxides 4 and 5 [3]. Ito reported [4] that phospholane derivatives 6 and 7 exhibited significant antitumor activities against leukemia cells such as the K562 and U937 cell lines, as well as solid cancer cells such as stomach cancer and lung cancer. Further mechanistic studies by Western blotting showed that such compounds could enhance the expression of IER5 and then suppressed the expression of Cdc25B, which are responsible for their antitumor activity [5].
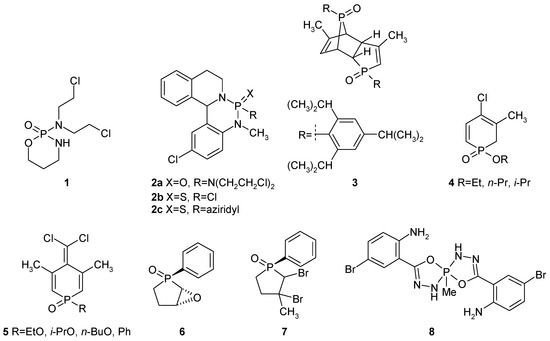
Figure 1.
Examples of phosphoheterocycles exhibiting anticancer activity 1–7 and the newly synthesized compound 8.
2. Results and Discussion
During our continuous efforts toward development of anticancer-relevant, heterocyclic derivatives [6,7,8], we investigated a reaction between 2-amino-5-bromobenzohydrazide 9, synthesized from 5-bromoisatoic anhydride and hydrazine hydrate according to the literature procedure [9], and methylphosphonyl dichloride 10 in the presence of triethylamine (Scheme 1). Equimolar amounts of 9 and 10 were dissolved in dry THF, three equivalents of triethylamine were added dropwise in room temperature and the reaction mixture was stirred in at room temperature for 18 h. Low-resolution mass spectrometry showed the formation of a new product with molecular mass M = 502 g/mol, and the isotopic profile revealed a product with two bromine atoms. To complete the reaction, one more equivalent of methylphosphonyl dichloride 10 and three more equivalents of triethylamine were added, and the reaction mixture was stirred at room temperature for an additional 18 h. This led to complete consumption of 9.
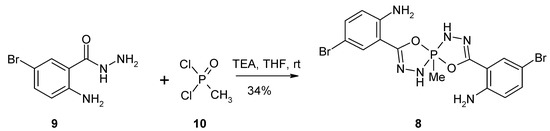
Scheme 1.
Synthesis of 5-methyl-3,8-di-(2-amino-4-bromophenyl)-4,9-dioxa-1,2,6,7-tetraaza-5λ5-phosphaspiro[4.4]nona-2,7-diene 8.
A literature search followed by the investigation of NMR spectra (see supplementary materials) led to a conclusion that product 8 possessed a 4,9-dioxa-1,2,6,7-tetraaza-5λ5-phosphaspiro[4.4]nona-2,7-diene core resulting from the condensation of two molecules of 2-amino-5-bromobenzohydrazide 9 with one molecule of methylphosphonyl dichloride 10. It was revealed that only the aromatic hydrazide group of 9 participated in the condensation with 10, while the primary aromatic amine group of 9 remained intact. Compounds possessing 4,9-dioxa-1,2,6,7-tetraaza-5λ5-phosphaspiro[4.4]nona-2,7-diene structure have been previously reported by Schmidpeter [10] and their synthesis has been more recently investigated by Gholivand [11] and Hua [12]. Similar condensations between aromatic dihydrazides and phosphonyl dichlorides were also published by Ali [13]. In previous reports, only simple aromatic hydrazides were condensed with phosphonyl dichlorides, which limited further modifications of obtained products. In contrast, the presence of two amine groups, as well as two bromide atoms within the structure of 8, allows further modifications of the initially obtained products. The structure of 8 allows for further elaboration to more complex molecules.
The necessity of adding the 2 equivalents of phosphonyl dichloride 10 and the relatively low yield of the reaction could be explained by the formation of other by-products. The organic phase obtained after extraction with ethyl acetate, contained the main, relatively non-polar product 8, and two minor, very polar and difficult to separate products, which have not been isolated and characterized thus far. Moreover, we cannot exclude a possibility that the water phase contained some additional polar products, insoluble in the organic phase. Our initial efforts to optimize the reaction conditions did not lead to a better yield or a more economic ratio of reagents. In addition, we recently found that although compound 8 is easily separated, purified, and characterized by physicochemical methods, its shelf-life (especially of the crude product before chromatographic purification), at room temperature, is rather limited. After the purification step, product 8 should be dried and stored at low temperature (−18 °C).
5-Methyl-3,8-di-(2-amino-4-bromophenyl)-4,9-dioxa-1,2,6,7-tetraaza-5λ5-phosphaspiro[4.4]nona-2,7-diene (8) was toxic to all studied cell lines: two cancer lines, epidermoid (A431) and glioblastoma (U87), as well as two non-cancer cell lines, embryonic kidney (HEK 293) and telomerase-immortalized fibroblasts (K21). The toxicity of the compound was similar for all cells and at 200 uM concentration survival dropped to about 25% for non-cancer cell lines, and to about 30% for cancer cell lines (Figure 2). Thus, compound 8 seems not to be a candidate for selective anticancer treatment.
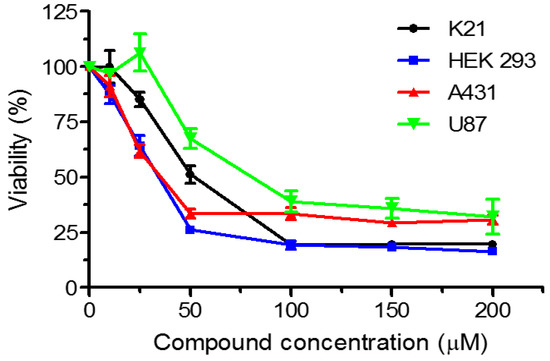
Figure 2.
Viability plots of cell lines K21, HEK 293, A431 and U87 in response to 5-methyl-3,8-di-(2-amino-4-bromophenyl)-4,9-dioxa-1,2,6,7-tetraaza-5λ5-phosphaspiro[4.4]nona-2,7-diene.
3. Materials and Methods
Commercially available chemicals were of reagent grade and used as received. The reactions were monitored by thin layer chromatography (TLC), using silica gel plates (Kieselgel 60F254, E. Merck, Darmstadt, Germany). Column chromatography was performed on silica gel 60M (0.040–0.063 mm, E. Merck, Darmstadt, Germany). Melting points are uncorrected and were measured on a Büchi (New Castle, DE, USA) Melting Point B-540 apparatus. The 1H, 13C and 31P-NMR spectra in CDCl3 were recorded at the Department of Chemistry, Warsaw University, using a Bruker AVANCE III HD (Billerica, MA, USA) 500 MHz spectrometer; shift values in parts per million are relative to the SiMe4 internal reference. The resonance assignments are based on a peak integration, peak multiplicity, and 2D correlation experiments. Multiplets were assigned as bs (broad singlet), d (doublet), dd (doublet of doublet) and tq (triplet of quartet). High-resolution mass spectra were recorded by the Laboratory of Mass Spectrometry, Institute of Biochemistry and Biophysics PAS, on a LTQ Orbitrap Velos instrument, Thermo Scientific (Waltham, MA, USA). IR spectra were recorded with a Jasco 6200 (Easton, MD, USA) FT/IR spectrometer at the Laboratory of Optical Spectroscopy, Institute of Organic Chemistry PAS (Warsaw, Poland).
Cytotoxic activity of 8 was verified against two cancer cell lines: A431 (human epidermoid carcinoma), U87 (human glioblastoma) and two non-cancer cell lines: K21 (human fibroblast) and HEK 293 (human embryonic kidney). Cells were seeded in 96-well plates at density of 3000 cells per well one day before treatment and then treated with increasing concentrations (10–200 µM) of tested compound in complete growth medium. After 48 h of incubation, cells were assayed to measure their viability using the alamarBlue assay (Invitrogen by Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s instructions. Each experiment was repeated three times.
Synthesis of 5-methyl-3,8-di-(2-amino-4-bromophenyl)-4,9-dioxa-1,2,6,7-tetraaza-5λ5-phosphaspiro[4.4]nona-2,7-diene 8.
2-Amino-5-bromobenzohydrazide (9, 500 mg, 2.17 mmol, 1 equiv.) and methylphosphonyl dichloride (10, 288 mg, 2.17 mmol, 1 equiv.) were dissolved in 15 mL of dry THF, then triethylamine (0.91 mL, 6.52 mmol, 3 equiv.) was added dropwise. The reaction mixture was stirred at room temperature for 18 h, then further portions of methylphosphonyl dichloride (10, 288 mg, 2.17 mmol, 1 equiv.) followed by triethylamine (0.91 mL, 6.52 mmol, 1 equiv.) were added, and the reaction mixture was stirred for another 18 h. After addition of 30 mL of water, the solution was extracted with ethyl acetate (3 × 30 mL). The organic phase was dried over magnesium sulfate, filtered and evaporated with silica gel (2 g). The final product was purified by column chromatography, using hexane:ethyl acetate 8:2 v/v mixture. The fractions containing 8 were collected, the solvent was evaporated under the reduced pressure giving a yellow oil, which solidified during overnight storage. The resulting yellow crystals were washed with methanol to give a white powder. Yield: 184 mg (34%). M.p. 229.5–230.5 °C. 1H-NMR (500 MHz, CDCl3): 7.66 (d, 2H, 4J = 2.5 Hz, HAr), 7.22 (dd, 2H, 4J = 2.5 Hz, 3J = 8.5 Hz, HAr), 6.57 (d, 2H, 3J = 8.5 Hz, HAr), 5.81 (d, 2H, 3J(H-P) = 31.0 Hz, 2 × NH); 5.43 (bs, 4H, 2 × NH2), 2.08 (d, 3H, 3J(H-P) = 18.0 Hz, CH3); 13C-NMR (125 MHz, CDCl3): 155.0 (d, 2JC-P = 10.2 Hz), 144.8, 133.1, 129.9, 117.4, 111.7 (d, 3JC-P = 0.8 Hz), 107.8, 22.9 (d, 1JC-P = 174.5 Hz); 31P-NMR (202 MHz, CDCl3): −34.45 (tq, 3J(H-P) = 31.0 Hz, 3J(H-P) = 18.0 Hz, coupled with 2 × NH and 1 × CH3); HRMS (ESI): m/z [M + H]+ calcd. for C15H15Br2N6O2P: 500.94336, 502.94132, 504.93927, found: 500.94359, 502.94151, 504.93945; IR (KBr): cm−1 3473, 3446, 3415, 3346, 2923, 2852, 1882, 1737, 1611, 1586, 1554, 1489, 1423, 1339, 1314, 1300, 1250, 1163, 1130, 1114, 1059.
Supplementary Materials
Copies of the 1H-NMR, 13C-NMR, dept135, 31P-NMR, IR, HRMS-ESI mass spectra are available online at http://www.mdpi.com/1422-8599/2018/1/M978/s1.
Acknowledgments
The equipment used for the synthesis was partially sponsored by the Centre for Preclinical Research and Technology (CePT), a project co-sponsored by the European Regional Development Fund and Innovative Economy, The National Cohesion Strategy of Poland. We thank Jacek Olędzki for recording the ES-MS spectra.
Author Contributions
A.M.: synthesis planning, writing of manuscript; S.K. experimental synthetic work; J.C., B.M.: screening of biological activity; B.T.: writing of manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ahmed, A.R.; Hombal, S.M. Cyclophosphamide (Cytoxan). A review on relevant pharmacology and clinical uses. J. Am. Acad. Dermatol. 1984, 11, 1115–1126. [Google Scholar] [CrossRef]
- Bull, E.O.J.; Naidu, M.S.R. Isoquino[2,1-c][1,3,2]benzodiazaphosphorine derivatives: New potential agents for cancer chemotherapy. Phosphorus Sulfur Silicon Relat. Elem. 2000, 162, 231–243. [Google Scholar] [CrossRef]
- Hudson, H.R.; Keglevich, G. The preparation and anticancer activity of some phosphorus heterocycles. Phosphorus Sulfur Silicon Relat. Elem. 2008, 183, 2256–2261. [Google Scholar] [CrossRef]
- Ito, S.; Yamashita, M.; Niimi, T.; Fujie, M.; Reddy, V.K.; Totsuka, H.; Haritha, B.; Maddali, K.; Nakamura, S.; Asai, K.; et al. Preparation and characterization of phospholanes and phosphasugars as novel anti-cancer agents. Heterocycl. Commun. 2009, 15, 23–30. [Google Scholar] [CrossRef]
- Hasegawa, H.; Yamashita, M.; Makita, R.; Yamaoka, M.; Fujie, M.; Nakamura, S.; Oshikawa, T.; Yamashita, J.; Yamada, M.; Kondo, M.; et al. Novel molecular targeted and wide spectrum antitumor agents: Preparation and preclinical evaluation of IER5/Cdc25B targeted low-molecular-weight phospha sugar derivatives. JJAP Conf. Proc. 2016, 4, 011301. [Google Scholar] [CrossRef]
- Mieczkowski, A.; Bazlekova, M.; Bagiński, M.; Wójcik, J.; Winczura, A.; Miazga, A.; Gajda, R.; Woźniak, K.; Tudek, B. A mild and efficient approach to 6H-oxazolo[3,2-f]pyrimidine-5,7-dione scaffold via unexpected rearrangement of 2,3-dihydropyrimido[6,1-b][1,5,3]dioxazepine-7,9(5H,8H)-diones: A synthesis, crystallographic studies and cytotoxic activity screening. Tetrahedron Lett. 2016, 57, 743–746. [Google Scholar] [CrossRef]
- Mieczkowski, A.; Trzebiński, D.; Wilczek, M.; Psurski, M.; Bagiński, M.; Bieszczad, B.; Mroczkowska, M.; Woźniak, K. (S)-2-(4-Chlorobenzoyl)-1,2,3,4-tetrahydrobenzo[e]pyrazino[1,2-a][1,4]diazepine-6,12(11H,12aH)-dione—Synthesis and Crystallographic Studies. Molbank 2017, 2017, M964. [Google Scholar] [CrossRef]
- Mieczkowski, A.; Wińska, P.; Kaczmarek, M.; Mroczkowska, M.; Garbicz, D.; Pilżys, T.; Marcinkowski, M.; Piwowarski, J.; Grzesiuk, E. 2′-Deoxy-2′-azidonucleoside analogues: Synthesis and evaluation of antitumor and antimicrobial activity. Chem. Pap. 2017. [Google Scholar] [CrossRef]
- Colotta, V.; Catarzi, D.; Varano, F.; Cecchi, L.; Filacchioni, G.; Galli, A.; Costagli, C. Synthesis and Biological Evaluation of a Series of Quinazoline-2-carboxylic Acids and Quinazoline-2,4-diones as Glycine-NMDA Antagonists: A Pharmacophore Model Based Approach. Arch. Pharm. 1997, 330, 129–134. [Google Scholar] [CrossRef]
- Schmidpeter, A.; Luber, J. Die produkte der Reaktion Carbonsäurehydrazid/Phosphonylchlorid: 2,2′-spirobi(1,3,4,2λ5-oxadiazaphospholine). Chem. Ber. 1977, 110, 1124–1129. [Google Scholar] [CrossRef]
- Gholivand, K.; Mahzouni, H.R.; Molami, F.; Kalateh, A.A. A phosphoryl to spiro-bicyclophosphorane transformation via β-amidic proton elimination in phosphorylated hydrazides. Tetrahedron Lett. 2012, 53, 5944–5947. [Google Scholar] [CrossRef]
- Hua, G.; Cordes, D.B.; Li, Y.; Slawin, A.M.Z.; Woollins, J.D. Symmetrical spiro-phosphoroheterocycles from selenation of carbohydrazides. Tetrahedron Lett. 2011, 52, 3311–3314. [Google Scholar] [CrossRef]
- Ali, T.E.; Halacheva, S.S. Synthetic Approach for Novel Bis(α-aminophosphonic acid) Derivatives of Chromone Containing 1,2,4,3-Triazaphosphole Moieties. Heteroat. Chem. 2009, 20, 117–122. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).