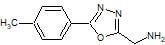
1-[5-(4-Tolyl)-1,3,4-oxadiazol-2-yl]methanamine
Abstract
1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. Materials
3.2. Instrumentaion
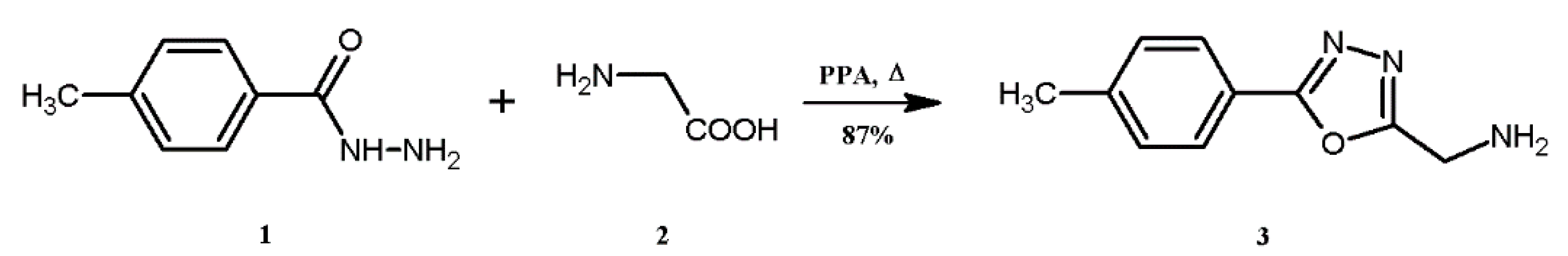
3.3. Synthesis of 1-[5-(4-Tolyl)-1,3,4-oxadiazol-2-yl]methanamine
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, C.; Zhao, P.; Huang, W. New oxadiazole derivatives as promising electron transport materials: Synthesis and characterization of thermal, optical and electrochemical properties. Cent. Eur. J. Chem. 2007, 5, 303–315. [Google Scholar] [CrossRef]
- Kaippamangalath, N.; Gopalakrishnapanicker, U. Synthesis and evaluation of properties of poly(p-phenylenevinylene) based 1,3,4-oxadiazole systems for optoelectronics and nonlinear optical applications. Polym. Int. 2016, 65, 1221–1231. [Google Scholar] [CrossRef]
- Chandrakantha, B.; Isloor, A.M.; Philip, R.; Mohesh, M.; Shetty, P.; Vijesh, A.M. Synthesis and nonlinear optical characterization of new 1,3,4-oxadiazoles. Bull. Mater. Sci. 2011, 34, 887–891. [Google Scholar] [CrossRef]
- Ichikawa, M.; Kawaguchi, T.; Kobayashi, K.; Miki, T.; Furukawa, K.; Koyama, T.; Taniguchi, Y. Bipyridyl oxadiazoles as efficient and durable electron-transporting and hole-blocking molecular materials. J. Mater. Chem. 2006, 16, 221–225. [Google Scholar] [CrossRef]
- Carli, S.; Baena, J.P.C.; Marianetti, G.; Marchetti, N.; Lessi, M.; Abate, A.; Caramori, S.; Grätzel, M.; Bellina, F.; Bignozzi, C.A.; et al. A New 1,3,4-Oxadiazole-Based Hole-Transport Material for Efficient CH3NH3PbBr3 Perovskite Solar Cells. ChemSusChem 2016, 9, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Chidirala, S.; Ulla, H.; Valaboju, A.; Kiran, M.R.; Mohanty, M.E.; Satyanarayan, M.N.; Umesh, G.; Bhanuprakash, K.; Rao, V.J. Pyrene–Oxadiazoles for Organic Light-Emitting Diodes: Triplet to Singlet Energy Transfer and Role of Hole-Injection/Hole-Blocking Materials. J. Org. Chem. 2016, 81, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Suwiński, J.; Szczepankiewicz, W. Five-membered Rings—Triazoles, Oxadiazoles, Thiadiazoles. In Comprehensive Heterocyclic Chemistry III; Katritzky, A.R., Ramsden, C.A., Scriven, E.F.V., Taylor, R.J.K., Eds.; Elseiver: Oxford, UK, 2008; Volume 5, Chapter 5.06; pp. 397–466. ISBN 9780080449920. [Google Scholar]
- Kim, J.H.; Lee, H. Enhancement of efficiency in luminescent polymer by incorporation of conjugated 1,3,4-oxadiazole side chains as hole-blocker/electron-transporter. Synth. Met. 2004, 143, 13–19. [Google Scholar] [CrossRef]
- Xu, X.; Li, Z.; Bi, Z.; Yu, T.; Ma, W.; Feng, K.; Li, Y.; Peng, Q. Highly Efficient Nonfullerene Polymer Solar Cells Enabled by a Copper(I) Coordination Strategy Employing an 1,3,4-Oxadiazole-Containing Wide-Bandgap Copolymer Donor. Adv. Mater. 2018, 30, 1800737. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, U.; Hussein, I.A.; Daud, M. New 1,3,4-Oxadiazole Based Photosensitizers for Dye Sensitized Solar Cells (DSSCs). Int. J. Photoenergy 2015, 2015. [Google Scholar] [CrossRef]
- Lü, Z.; Deng, Z.; Zheng, J.; Xu, D.; Chen, Z.; Zhou, E.; Wang, Y. Organic light-emitting diodes with 2-(4-biphenylyl)-5(4-tert-butyl-phenyl)-1,3,4-oxadiazole layer inserted between hole-injecting and hole-transporting layers. Vacuum 2010, 84, 1287–1290. [Google Scholar] [CrossRef]
- Ma, D.; Lupton, J.M.; Samuel, D.W.; Lo, S.C.; Burn, P.L. Bright electroluminescence from a conjugated dendrimer. Appl. Phys. Lett. 2002, 81, 2285–2287. [Google Scholar] [CrossRef]
- Paun, A.; Hadade, N.D.; Paraschivescu, C.C.; Matache, M. 1,3,4-Oxadiazoles as luminescent materials for organic light emitting diodes via cross-coupling reactions. J. Mater. Chem. C 2016, 4, 8596–8610. [Google Scholar] [CrossRef]
- Kim, S.J.; Zhang, Y.; Zuniga, C.; Barlow, S.; Marder, S.R.; Kippelen, B. Efficient green OLED devices with an emissive layer comprised of phosphor-doped carbazole/bis-oxadiazole side-chain polymer blends. Org. Electron. 2011, 12, 492–496. [Google Scholar] [CrossRef]
- Ichikava, M.; Shimizu, C.; Koyama, T.; Taniguchi, Y. Improvement of photovoltaic performances of organic thin-film solar cells by fast electron mobility oxadiazole as an exciton blocking layer material. Phys. Stat. Sol. 2008, 205, 1222–1225. [Google Scholar] [CrossRef]
- Mansoori, Y.; Ghanbari, M. Novel polyimides obtained from a new aromatic diamine (BAPO) containing pyridine and 1,3,4-oxadiazole moieties for removal of Co(II) and Ni(II) ions. Polym. Adv. Technol. 2015, 26, 658–664. [Google Scholar] [CrossRef]
- Mercer, F.W.; McKenzie, M.T. Dielectric and thermal characterization of fluorinated polyimides containing heterocyclic moieties. High Perform. Polym. 1993, 5, 97–106. [Google Scholar] [CrossRef]
- Ganesh, S.D.; Pai, V.K.; Kariduraganavar, M.Y.; Jayanna, M.B. Functional Aromatic Poly(1,3,4-Oxadiazole-Ether)s with Benzimidazole Pendants: Synthesis, Thermal and Dielectric Studies. Int. Sch. Res. Not. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, S.D.; Pai, V.K.; Kariduraganavar, M.Y.; Jayanna, M.B. Thermal and Dielectric Behavior Studies of Poly(Arylene Ether Sulfone)s with Sulfonated and Phosphonated Pendants. J. Mater. 2016, 2016. [Google Scholar] [CrossRef]
- Bruma, M.; Hamciuc, E.; Schulz, B.; Kopnick, T.; Kaminorz, Y.; Robison, J. Synthesis and study of new polyamides with side oxadiazole rings. J. Appl. Polym. Sci. 2003, 87, 714–721. [Google Scholar] [CrossRef]
- Tasaganva, R.G.; Tambe, S.M.; Kariduraganavar, M.Y. Synthesis and characterization of thermally stable second-order nonlinear optical side-chain polyurethanes containing nitro-substituted oxadiazole and thiazole chromophores. J. Mol. Struct. 2011, 1000, 10–23. [Google Scholar] [CrossRef]
- Tasaganva, R.G.; Doddamani, R.V.; Inamdar, S.R.; Kariduraganavar, M.Y. Synthesis of thermally stable new polyurethanes containing nitro-substituted 1,3,4-oxadiazole chromophores for second order nonlinear optical applications. Optik 2015, 126, 4991–5000. [Google Scholar] [CrossRef]
- Sun, J.; Makawana, J.A.; Zhu, H.L. 1,3,4-Oxadiazole Derivatives as Potential Biological Agents. Mini-Rev. Med. Chem. 2013, 13, 1725–1743. [Google Scholar] [CrossRef] [PubMed]
- Shridhar, A.H.; Keshavayya, J.; Peethambar, S.K.; Hoskeri, H.J. Synthesis and biological activities of Bis alkyl 1,3,4-oxadiazole incorporated azo dye derivatives. Arabian J. Chem. 2016, 9, S1643–S1648. [Google Scholar] [CrossRef]
- Kadi, A.A.; El-Brollosy, N.R.; Al-Deeb, O.A.; Habib, E.E.; Ibrahim, T.M.; El-Emam, A.A. Synthesis, antimicrobial, and anti-inflammatory activities of novel 2-(1-adamantyl)-5-substituted-1,3,4-oxadiazoles and 2-(1-adamantylamino)-5-substituted-1,3,4-thiadiazoles. Eur. J. Med. Chem. 2007, 42, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Weaver, G.W. Product Class 8: 1,3,4-Oxadiazoles. In Science of Synthesis; Storr, R.C., Gilchrist, T.L., Eds.; Georg Thieme: Stuttgart, Germany, 2003; Volume 13, Chapter 13.8; pp. 219–252. [Google Scholar]
- Mickevičius, V.; Vaickelioniene, R.; Sapijanskaite, B. Synthesis of substituted 1,3,4-oxadiazole derivatives. Chem. Heterocycl. Compd. 2009, 45, 215–218. [Google Scholar] [CrossRef]
- Gomes, D.; Borges, C.P.; Pinto, J.C. Study of the synthesis of poly(4,4′-diphenylether-1,3,4-oxadiazole) in solutions of poly(phosphoric acid). Polymer 2001, 42, 851–865. [Google Scholar] [CrossRef]
- Pidugu, V.R.; Yarla, N.S.; Pedada, S.R.; Kalle, A.M.; Satya, A.K. Design and synthesis of novel HDAC8 inhibitory 2,5-disubstituted-1,3,4-oxadiazoles containing glycine and alanine hybrids with anticancer activity. Bioorg. Med. Chem. 2016, 24, 5611–5617. [Google Scholar] [CrossRef] [PubMed]
- Poddubnyi, I.S.; Belen’kii, L.I.; Struchkova, M.I.; Krayushkin, M.M. 1H and 13C NMR spectra of 1,5-disubstituted 1,3,4-oxadiazoles. Chem. Heterocycl. Compd. 1994, 30, 729–737. [Google Scholar] [CrossRef]
- Desai, N.C.; Dodiya, A.M.; Rajpara, K.M.; Rupala, Y.M. Synthesis and antimicrobial screening of 1,3,4-oxadiazole and clubbed thiophene derivatives. J. Saudi Chem. Soc. 2014, 18, 255–261. [Google Scholar] [CrossRef]
- Kim, B.S.; Ahn, S.; Koh, D.; Cho, S.M.; Song, Y.W.; Sung, J.; Lim, Y. 1H and 13C NMR characterization of 1,3,4-oxadiazole derivatives. Magn. Reson. Chem. 2018, 56, 782–791. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimoga, G.; Shin, E.-J.; Kim, S.-Y. 1-[5-(4-Tolyl)-1,3,4-oxadiazol-2-yl]methanamine. Molbank 2018, 2018, M1014. https://doi.org/10.3390/M1014
Shimoga G, Shin E-J, Kim S-Y. 1-[5-(4-Tolyl)-1,3,4-oxadiazol-2-yl]methanamine. Molbank. 2018; 2018(3):M1014. https://doi.org/10.3390/M1014
Chicago/Turabian StyleShimoga, Ganesh, Eun-Jae Shin, and Sang-Youn Kim. 2018. "1-[5-(4-Tolyl)-1,3,4-oxadiazol-2-yl]methanamine" Molbank 2018, no. 3: M1014. https://doi.org/10.3390/M1014
APA StyleShimoga, G., Shin, E.-J., & Kim, S.-Y. (2018). 1-[5-(4-Tolyl)-1,3,4-oxadiazol-2-yl]methanamine. Molbank, 2018(3), M1014. https://doi.org/10.3390/M1014