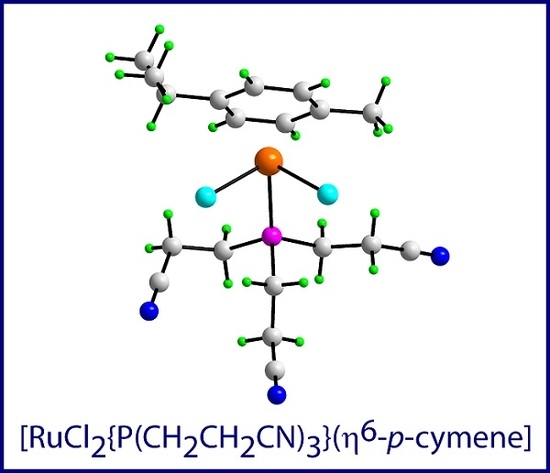
Dichlorido(η6-p-cymene)[tris(2-cyanoethyl)phosphine]ruthenium(II)
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Synthesis of [RuCl2{P(CH2CH2CN)3}(η6-p-cymene)]
3.3. Crystallography
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vullo, W.J. Hydroxymethyl replacement reactions of tetrakis(hydroxymethyl)phosphonium chloride. Ind. Eng. Chem. Prod. Res. Dev. 1966, 5, 346–349. [Google Scholar] [CrossRef]
- Cooney, K.D.; Cundari, T.R.; Hoffman, N.W.; Pittard, K.A.; Temple, M.D.; Zhao, Y. A priori assessment of the stereoelectronic profile of phosphines and phosphites. J. Am. Chem. Soc. 2003, 125, 4318–4324. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.L.; Lee, K.J. Ligand steric properties. Coord. Chem. Rev. 1993, 128, 89–116. [Google Scholar] [CrossRef]
- Fisher, K.J.; Henderson, W.; Dance, I.G.; Willett, G.D. Investigation of the properties of tris(2-cyanoethyl)phosphine by electrospray and Fourier transform ion cyclotron resonance mass spectrometries. J. Chem. Soc. Dalton Trans. 1996, 4109–4113. [Google Scholar] [CrossRef]
- Orpen, A.G.; Pringle, P.G.; Smith, M.B.; Worboys, K. Synthesis and properties of new tris(cyanoethyl)phosphine complexes of platinum(0,II), palladium(0,II), iridium(I) and rhodium(I). Conformational analysis of tris(cyanoethyl)phosphine ligands. J. Organomet. Chem. 1998, 550, 255–266. [Google Scholar] [CrossRef]
- Costa, E.; Pringle, P.G.; Smith, M.B.; Worboys, K. Self-replication of tris(cyanoethyl)phosphine catalyzed by platinum group metal complexes. J. Chem. Soc. Dalton Trans. 1997, 4277–4282. [Google Scholar] [CrossRef]
- Lawrence, S.E.; Kelly, M.; Ni Dhubhghaill, O.M. Dichloro-trans-bis[tris(2-cyanoethyl)phosphine] palladium(II). Acta Crystallogr. E 2001, 57, m272–m273. [Google Scholar] [CrossRef]
- Hussain, M.S.; Al-Arfaj, A.R.; Akhtar, M.N.; Isab, A.A. [{(CEP)2Au}+{Au(CN)2}-]: A compound with gold-gold bonds. Polyhedron 1996, 15, 2781–2785. [Google Scholar] [CrossRef]
- Pruchnik, F.P.; Smolenski, P.; Raksa, I. Carbonyl rhodium(I) and ruthenium(II) complexes with water soluble phosphines. Pol. J. Chem. 1995, 69, 5–8. [Google Scholar]
- Ballester-Reventos, L.; Gutierrez-Alonso, A.; Perpiñan-Vielba, M.F. Formation of heterobimetallic ruthenium-mercury nitrosyl complexes. Polyhedron 1991, 10, 1013–1017. [Google Scholar] [CrossRef]
- Chen, L.; Poë, A.J. Systematic substituent effects on dissociative substitution kinetics of ruthenium carbonyl, Ru(CO)4L, complexes (L = phosphorus, arsenic, and antimony donor ligands). Inorg. Chem. 1989, 28, 3641–3647. [Google Scholar] [CrossRef]
- Bruce, M.I.; Matisons, J.G.; Nicholson, B.K. Cluster chemistry. XVII. Radical ion-initiated syntheses of ruthenium cluster carbonyls containing tertiary phosphines, phosphites, arsines, SbPh3 or isocyanides. J. Organomet. Chem. 1983, 247, 321–343. [Google Scholar] [CrossRef]
- Gutierrez-Alonso, A.; Ballester-Reventos, L. Reactions of carbonyl and nitrosyl ruthenium complexes with uninegative (X,S)-donor ligands (X = sulfur, phosphorus). Polyhedron 1991, 10, 1019–1023. [Google Scholar] [CrossRef]
- Gutiérrez Alonso, A.; Ballester Reventós, L. Cyclopentadienylruthenium complexes with sulfur donor ligands. II. A comparative study of the reactivity of Ru(η-RC5H4)Cl(L)2 (R = H, CH3, CH3CO; L = CO, Ph2PCH2CH2PPh2/2, P(CH2CH2CN)3) towards anionic (S-S) donor ligands. J. Organomet. Chem. 1988, 338, 249–254. [Google Scholar] [CrossRef]
- Jensen, S.B.; Rodger, S.J.; Spicer, M.D. Facile preparation of η6-p-cymene ruthenium diphosphine complexes. Crystal structure of [(η6-p-cymene)Ru(dppf)Cl]PF6. J. Organomet. Chem. 1998, 556, 151–158. [Google Scholar] [CrossRef]
- Henderson, W.; Evans, C. Electrospray mass spectrometric analysis of transition-metal halide complexes. Inorg. Chim. Acta 1999, 294, 183–192. [Google Scholar] [CrossRef]
- Fridgen, T.D.; Burkell, J.L.; Wilsily, A.N.; Braun, V.; Wasylycia, J.; McMahon, T.B. Potential energy surfaces for gas-phase SN2 reactions involving nitriles and substituted nitriles. J. Phys. Chem. A 2005, 109, 7519–7526. [Google Scholar] [CrossRef] [PubMed]
- Strohalm, M.; Hassman, M.; Košata, B.; Kodíček, M. mMass data miner: An open source alternative for mass spectrometric data analysis. Rapid Commun. Mass Spectrom. 2008, 22, 905–908. [Google Scholar] [CrossRef] [PubMed]
- Rigaku Oxford Diffraction. CrysAlis PRO; Agilent Technologies Inc.: Santa Clara, CA, USA, 2015. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Brandenburg, K.; Putz, H. DIAMOND; Crystal Impact GbR: Bonn, Germany, 2006. [Google Scholar]
Sample Availability: A sample of the complex is not available from the authors. |



| A | H | B | A–H | H⋯B | A⋯B | A–H⋯B | Symmetry Operation |
|---|---|---|---|---|---|---|---|
| C1 | H1b | Cl2 | 0.99 | 2.80 | 3.738(2) | 158 | x, 1 + y, z |
| C7 | H7a | Cl1 | 0.99 | 2.73 | 3.711(2) | 170 | x, 1 + y, z |
| C14 | H14 | Cl1 | 0.95 | 2.80 | 3.446(2) | 126 | x, 1 + y, z |
| C15 | H15 | Cl1 | 0.95 | 2.83 | 3.461(2) | 125 | x, 1 + y, z |
| C15 | H15 | Cl2 | 0.95 | 2.68 | 3.5752(19) | 157 | x, 1 + y, z |
| C5 | H5b | N1 | 0.99 | 2.51 | 3.402(3) | 150 | 1 − x, 2 − y, −z |
| C12 | H12 | N2 | 0.95 | 2.57 | 3.289(3) | 132 | ½ + x, ½ − y, ½ + z |
| C19 | H19c | N3 | 0.98 | 2.69 | 3.636(3) | 126 | ½ + x, 1½ − y, ½ + z |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henderson, W.; Nair, A.G.; Halcovich, N.R.; Tiekink, E.R.T. Dichlorido(η6-p-cymene)[tris(2-cyanoethyl)phosphine]ruthenium(II). Molbank 2018, 2018, M1025. https://doi.org/10.3390/M1025
Henderson W, Nair AG, Halcovich NR, Tiekink ERT. Dichlorido(η6-p-cymene)[tris(2-cyanoethyl)phosphine]ruthenium(II). Molbank. 2018; 2018(4):M1025. https://doi.org/10.3390/M1025
Chicago/Turabian StyleHenderson, William, Ashwin Gopalan Nair, Nathan R. Halcovich, and Edward R. T. Tiekink. 2018. "Dichlorido(η6-p-cymene)[tris(2-cyanoethyl)phosphine]ruthenium(II)" Molbank 2018, no. 4: M1025. https://doi.org/10.3390/M1025