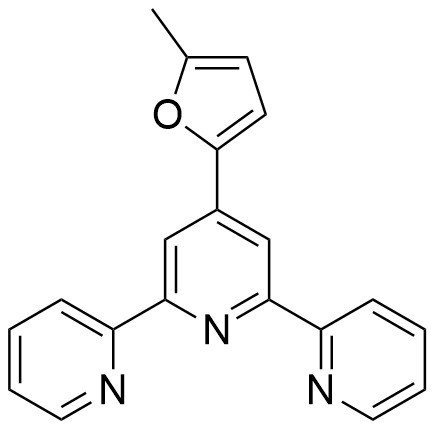
4′-(5-Methylfuran-2-yl)-2,2′:6′,2″-terpyridine: A New Ligand Obtained from a Biomass-Derived Aldehyde with Potential Application in Metal-Catalyzed Reactions
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Preparation of 4′-(5-Methylfuran-2-yl)-2,2′:6′,2″-terpyridine
3.2. Nickel-Catalyzed Dimerization of Benzyl Bromide with Compound 1 as a Ligand
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Schubert, U.S.; Hofmeier, H.; Newkome, G.R. Modern Terpyridine Chemistry; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Saccone, D.; Magistris, C.; Barbero, N.; Quagliotto, P.; Barolo, C.; Viscardi, G. Terpyridine and Quaterpyridine Complexes as Sensitizers for Photovoltaic Applications. Materials 2016, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Naidji, B.; Husson, J.; Et Taouil, A.; Brunol, E.; Sanchez, J.-B.; Berger, F.; Rauch, J.-Y.; Guyard, L. Terpyridine-based metallopolymer thin films as active layer in ammonia sensor device. Synth. Met. 2016, 221, 214–219. [Google Scholar] [CrossRef]
- Cummings, S.D. Platinum complexes of terpyridine: interaction and reactivity with biomolecules. Coord. Chem. Rev. 2009, 253, 1495–1516. [Google Scholar] [CrossRef]
- Young, D.C.; Yang, H.; Telfer, S.G.; Kruger, P.E. An Isoreticular Series of Zinc(II) Metal-Organic Frameworks Derived from Terpyridylcarboxylate Ligands. Inorg. Chem. 2017, 56, 12224–12231. [Google Scholar] [CrossRef] [PubMed]
- Heller, M.; Schubert, U.S. Syntheses of functionalized 2,2’:6’,2’’-terpyridines. Eur. J. Org. Chem. 2003, 6, 947–961. [Google Scholar] [CrossRef]
- Fallahpour, R.A. Synthesis of 4′-substituted-2,2’:6’,2’’-terpyridines. Synthesis 2003, 2, 155–184. [Google Scholar] [CrossRef]
- Thompson, A.M.W.C. The synthesis of 2,2’:6’,2’’-terpyridine ligands- versatile building blocks for supramolecular chemistry. Coord. Chem. Rev. 1997, 160, 1–52. [Google Scholar] [CrossRef]
- Kröhnke, F. The Specific Synthesis of Pyridines and Oligopyridines. Synthesis 1976, 1, 1–24. [Google Scholar] [CrossRef]
- Sasaki, I. Recent Uses of Kröhnke Methodology: A Short Survey. Synthesis 2016, 48, 1974–1992. [Google Scholar] [CrossRef]
- Husson, J.; Knorr, M. Syntheses and applications of furanyl-functionalised 2,2′:6′,2′′-terpyridines. Beilstein J. Org. Chem. 2012, 8, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Delbecq, F.; Wang, Y.; Muralidhara, A.; Ei Ouardi, K.; Marlair, G.; Len, C. Hydrolysis of Hemicellulose and Derivatives—A Review of Recent Advances in the Production of Furfural. Front. Chem. 2018, 6, 146. [Google Scholar] [CrossRef] [PubMed]
- Dehaudt, J.; Husson, J.; Guyard, L. A more efficient synthesis of 4,4’,4’’-tricarboxy-2,2’:6’,2’’-terpyridine. Green Chem. 2011, 13, 3337–3340. [Google Scholar] [CrossRef]
- Yang, W.; Sen, A. Direct Catalytic Synthesis of 5-Methylfurfural from Biomass-Derived Carbohydrates. ChemSusChem 2011, 4, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hanan, G.S. A Facile Route to Sterically Hindered and Non-Hindered 4’-Aryl-2,2’:6’,2’’-Terpyridines. Synlett 2005, 8, 1251–1254. [Google Scholar] [CrossRef]
- Winter, A.; Newkome, G.R.; Schubert, U.S. Catalytic Applications of Terpyridines and their Transition Metal Complexes. ChemCatChem 2011, 3, 1384–1406. [Google Scholar] [CrossRef]
- Anderson, T.J.; Jones, G.D.; Vicic, D.A. Evidence for a NiI Active Species in the Catalytic Cross-Coupling of Alkyl Electrophiles. J. Am. Chem. Soc. 2004, 126, 8100–8101. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.D.; Martin, J.L.; McFarland, C.; Allen, O.R.; Hall, R.E.; Haley, A.D.; Brandon, R.J.; Konovalova, T.; Desrochers, P.J.; Pulay, P.; Vicic, D.A. Ligand Redox Effects in the Synthesis, Electronic Structure, and reactivity of an Alkyl-Alkyl Cross-Coupling Catalyst. J. Am. Chem. Soc. 2006, 128, 13175–13183. [Google Scholar] [CrossRef] [PubMed]
- Prinsell, M.R.; Everson, D.A.; Weix, D.J. Nickel-catalyzed, sodium iodide-promoted reductive dimerization of alkyl halides, alkyl pseudohalides, and allylic acetates. Chem. Commun. 2010, 46, 5743–5745. [Google Scholar] [CrossRef] [PubMed]
- Huihui, K.M.M.; Shrestha, R.; Weix, D.J. Nickel-Catalyzed Reductive Conjugate Addition of Primary Alkyl Bromides to Enones To Form Silyl Enol Ethers. Org. Lett. 2017, 19, 340–343. [Google Scholar] [CrossRef] [PubMed]
- Felpin, F.-X.; Fouquet, E. A Useful, Reliable and Safer Protocol for Hydrogenation and the Hydrogenolysis of O-Benzyl Groups: The In Situ Preparation of an Active Pd0/C Catalyst with Well-Defined Properties. Chem. Eur. J. 2010, 16, 12440–12445. [Google Scholar] [CrossRef] [PubMed]



| Ligand | Yield (%) |
|---|---|
| 4,4′,4″-tritertbutyl-2,2′:6′,2″-terpyridine | 81 |
| 1 | 2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Husson, J.; Guyard, L. 4′-(5-Methylfuran-2-yl)-2,2′:6′,2″-terpyridine: A New Ligand Obtained from a Biomass-Derived Aldehyde with Potential Application in Metal-Catalyzed Reactions. Molbank 2018, 2018, M1032. https://doi.org/10.3390/M1032
Husson J, Guyard L. 4′-(5-Methylfuran-2-yl)-2,2′:6′,2″-terpyridine: A New Ligand Obtained from a Biomass-Derived Aldehyde with Potential Application in Metal-Catalyzed Reactions. Molbank. 2018; 2018(4):M1032. https://doi.org/10.3390/M1032
Chicago/Turabian StyleHusson, Jérôme, and Laurent Guyard. 2018. "4′-(5-Methylfuran-2-yl)-2,2′:6′,2″-terpyridine: A New Ligand Obtained from a Biomass-Derived Aldehyde with Potential Application in Metal-Catalyzed Reactions" Molbank 2018, no. 4: M1032. https://doi.org/10.3390/M1032