Solution Equilibria Formation of Manganese(II) Complexes with Ethylenediamine, 1,3-Propanediamine and 1,4-ButanediaMine in Methanol
Abstract
:1. Introduction
2. Results and Discussion
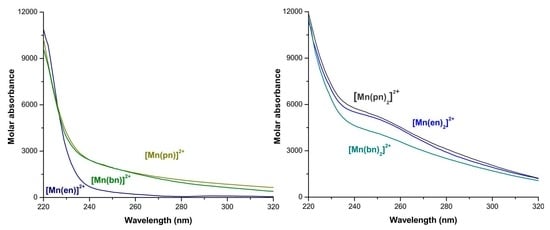
2.1. Formation Constants of Manganese(II) Systems with Ethylenediamine, 1,3-Propanediamine or 1,4-Butanediamine
2.2. Distribution Curves of the Manganese(II) with Ethylenediamine, 1,3-Propanediamine or 1,4-Butanediamine Systems
3. Experimental Section
3.1. Materials, Physical Measurements and Methods
3.2. Equilibrium Studies of the Manganese(II) with Ethylenediamine, 1,3-Propanediamine and 1,4-Butanediamine
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salomon, E.; Keren, N.; Kanteev, M.; Adir, N. Manganese in Biological Systems: Transport and Function in PATAI’S Chemistry of Functional Groups. Chem. Organomanganese Compd. 2011, 1–16. [Google Scholar] [CrossRef]
- Pfxoraro, V.L. Structural proposals for the manganese centers of the oxygen evolving complex: An inorganic chemists perspective. Photochem. Photobiol. 1988, 48, 249–264. [Google Scholar] [CrossRef] [Green Version]
- Bertini, I. Bioinorganic Chemistry; University Science Books: Mill Valley, CA, USA, 1994. [Google Scholar]
- Joliot, P.; Barbieri, G.; Chabaud, R. Un nouveau modele des centers photochimiques du systeme II. Photochem. Photobiol. 1969, 10, 309–329. [Google Scholar] [CrossRef]
- McConnell, I.; Li, G.; Brudvig, G.W. Energy Conversion in Natural and Artificial Photosynthesis. Chem. Biol. 2010, 17, 434–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stochel, G.; Stasicka, Z.; Brindell, M.; Macyk, W.; Szacilowski, K. Bioinorganic Photochemistry; Wiley: West Sussex, UK, 2009. [Google Scholar]
- Nelson, N.; Ben-Shem, A. The complex architecture of oxygenic photosynthesis. Nat. Rev. Mol. Cell Biol. 2004, 5, 971–982. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Högblom, J.; Anderlund, M.F.; Sun, L.; Magnuson, A.; Styring, S. Light-induced multistep oxidation of dinuclear manganese complexes for artificial photosynthesis. J. Inorg. Biochem. 2004, 98, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, J.; Ananyev, G.M.; Dismukes, G.C. Photoassembly of the Water-Oxidizing Complex in Photosystem II. Coord. Chem. Rev. 2008, 252, 347–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, F.A. Why did Nature choose manganese to make oxygen? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008, 363, 1263–1270. [Google Scholar] [CrossRef] [Green Version]
- Alstrum-Acevedo, J.H.; Brennaman, M.K.; Meyer, T.J. Chemical Approaches to Artificial Photosynthesis. 2. Inorg. Chem. 2005, 44, 6802–6827. [Google Scholar] [CrossRef] [Green Version]
- Han, G.; Li, J.; Chen, G.; Ling, L.; Li, S.; Khorobrykh, A.A.; Zharmukhamedov, S.K.; Klimov, V.V.; Kuang, T. Reconstruction of the water-oxidizing complex in manganese-depleted Photosystem II using synthetic manganese complexes. J. Photochem. Photobiol. B Biol. 2005, 81, 114–120. [Google Scholar] [CrossRef]
- Poulsen, A.K.; Rompel, A.; McKenzie, C.J. Water Oxidation Catalyzed by a Dinuclear Mn Complex: A Functional Model for the Oxygen-Evolving Center of Photosystem II. Angew. Chem. Int. Ed. 2005, 44, 6916–6920. [Google Scholar] [CrossRef]
- Magnuson, A.; Liebisch, P.; Högblom, J.; Anderlund, M.; Lomoth, R.; Meyer-Klaucke, W.; Haumann, M.; Dau, H. Bridging-type changes facilitate successive oxidation steps at about 1 V in two binuclear manganese complexes—Implications for photosynthetic water-oxidation. J. Inorg. Biochem. 2006, 100, 1234–1243. [Google Scholar] [CrossRef]
- Cady, C.W.; Crabtree, R.H.; Brudvig, G.W. Functional Models for the Oxygen-Evolving Complex of Photosystem II. Coord. Chem. Rev. 2008, 252, 444–455. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Chen, C.; Dong, H.; Shen, J.-R.; Dau, H.; Zhao, J. A synthetic Mn4Ca-cluster mimicking the oxygen-evolving center of photosynthesis. Science 2015, 348, 690–693. [Google Scholar] [CrossRef]
- Collomb, M.-N.; Deronzier, A. Manganese: Inorganic & Coordination Chemistry. In Encyclopedia of Inorganic Chemistry; John Wiley & Sons, Ltd.: West Sussex, UK, 2006. [Google Scholar]
- Pal, S.; Pal, S. Syntheses, structures and properties of trans-dichlororuthenium(II) complexes with N4-donor Schiff bases. Polyhedron 2003, 22, 867–873. [Google Scholar] [CrossRef]
- Kirk, A.D.; Ibrahim, A.M. Wavelength dependence of unquenchable photoaquation in the tris(1,3-diaminopropane)chromium(III) ion. Inorg. Chem. 1990, 29, 4848–4850. [Google Scholar] [CrossRef]
- González, J.G.; Nájera-Lara, M.; López-Ramírez, V.; Ramírez-Vázquez, J.A.; Segoviano-Garfias, J.J. Spectrophotometric determination of the formation constants of Calcium(II) complexes with 1,2-ethylenediamine, 1,3-propanediamine and 1,4-butanediamine in acetonitrile. Green Energy Environ. 2017, 2, 51–57. [Google Scholar] [CrossRef]
- Segoviano-Garfias, J.J.N.; Zanor, G.A.; Ávila-Ramos, F.; Bivián-Castro, E.Y. Stability of Manganese(II)-Pyrazine, -Quinoxaline or -Phenazine Complexes and Their Potential as Carbonate Sequestration Agents. Molecules 2022, 27, 1648. [Google Scholar] [CrossRef]
- Douglas, B.E.; Alexander, J.J. Conceptos y Modelos de Química Inorgánica; Reverté: Barcelona, Spain, 1994. [Google Scholar]
- Segoviano-Garfias, J.J.N.; Mendoza-Díaz, G.; Moreno-Esparza, R. Spectrophotometric determination of the formation constants of the cupric halogen complexes with 1,3-propanediamine and 1,4-butanediamine in methanol solution and their activity on the oxidative coupling of the 2,6-di-tert-butylphenol. Inorg. Chim. Acta 2013, 400, 184–190. [Google Scholar] [CrossRef]
- Tuck, D.G. A proposal for the use of a standard format for the publication of stability constant measurements. Pure Appl. Chem. 1989, 61, 1161. [Google Scholar] [CrossRef]
- Van Eldik, R.; Reedijk, J. Advances in Inorganic Chemistry: Homogeneous Biomimetic Oxidation Catalysis; Elsevier Science: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Oms, M.; Forteza, R.; Cerdá, V.; Borrull, F. Thermometric study of metal-nitrogenated-ligand complexes. i. ethylenediamine complexes with Ag(I), Cu(II), Zn(II), Ni(II), Mn(II), Cd(II) and Co(II). Thermochim. Acta 1989, 138, 1–12. [Google Scholar] [CrossRef]
- Mui, K.-K.; McBryde, W.A.E.; Nieboer, E. The Stability of Some Metal Complexes in Mixed Solvents. Can. J. Chem. 1974, 52, 1821–1833. [Google Scholar] [CrossRef]
- Pool, K.H.; Sandberg, D.E. Potentiometric determination of successive stability constants of ethylenediamine complexes of several metals in dimethylsulphoxide. Talanta 1969, 16, 1319–1323. [Google Scholar] [CrossRef]
- Marcus, Y. The effectivity of solvents as electron pair donors. J. Solut. Chem. 1984, 13, 599–624. [Google Scholar] [CrossRef]
- Mayer, U. Solvent effects on ion-pair equilibria. Coord. Chem. Rev. 1976, 21, 159–179. [Google Scholar] [CrossRef]
- Fan, J.; Shen, X.; Wang, J. Determination of Stability Constants of Copper(II)-Glycine Complex in Mixed Solvents by Copper(II)-Selective Electrode. Electroanalysis 2001, 13, 1115–1118. [Google Scholar] [CrossRef]
- Gorelsky, S.I.; Basumallick, L.; Vura-Weis, J.; Sarangi, R.; Hodgson, K.O.; Hedman, B.; Fujisawa, K.; Solomon, E.I. Spectroscopic and DFT Investigation of [M{HB(3,5-(i)Pr(2)pz)(3)}(SC(6)F(5))] (M = Mn, Fe, Co, Ni, Cu, and Zn) Model Complexes: Periodic Trends in Metal-thiolate Bonding. Inorg. Chem. 2005, 44, 4947–4960. [Google Scholar] [CrossRef] [Green Version]
- Arifin, K.; Majlan, E.H.; Daud, W.R.W.; Kassim, M.B. Bimetallic complexes in artificial photosynthesis for hydrogen production: A review. Int. J. Hydrogen Energy 2011, 37, 3066–3087. [Google Scholar] [CrossRef]
- Miessler, G.L.; Tarr, D.A. Inorganic Chemistry; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2004. [Google Scholar]
- Pérez-Arredondo, M.d.; González-Ponce, M.d.; Zanor, G.A.; Vazquez, J.A.R.; Segoviano-Garfias, J.J.N. Complex formation equilibria of 2,2′-bipyridyl and 1,10-phenanthroline with manganese(II) in methanol. Karbala Int. J. Mod. Sci. 2015, 1, 178–186. [Google Scholar] [CrossRef] [Green Version]
- Melník, M.; Chemikovna, S.S. New Trends in Coordination, Bioinorganic and Applied Inorganic Chemistry: XXIII. In Proceedings of the International Conference on Coordination and Bioinorganic Chemistry, Smolenice, Slovakia, 5–10 June 2011; Book of Abstracts, Program; Slovak University of Technology Press: Bratislava, Slovakia, 2011. [Google Scholar]
- Rocha, J.C.D.; Poneti, G.; Ferreira, J.G.; Ribeiro, R.R.; Nunes, F.S. Spectroscopic, Electrochemical, Magnetic and Structural Investigations of Dimanganese-(II/II) and Mixed-Valence-(II/III)-Tetraiminodiphenolate Complexes. J. Braz. Chem. Soc. 2014, 25, 1528–1535. [Google Scholar] [CrossRef]
- Halverson, F.; Hirt, R.C. Near Ultraviolet Solution Spectra of the Diazines. J. Chem. Phys. 1951, 19, 711–718. [Google Scholar] [CrossRef]
- Hirt, R.C. and R.G. Schmitt, Observed n − π* Bands in the Ultraviolet Absorption Solution Spectra of Amino Asymmetric Triazines. J. Chem. Phys. 1955, 23, 600. [Google Scholar] [CrossRef]
- Ito, M.; Shimada, R.; Kuraishi, T.; Mizushima, W. Ultraviolet Absorption of Pyrazine Vapor Due to n − π Transition. J. Chem. Phys. 1957, 26, 1508–1515. [Google Scholar] [CrossRef]
- Lavergne, J. Improved UV-visible spectra of the S-transitions in the photosynthetic oxygen-evolving system. Biochim. Biophys. Acta (BBA) Bioenerg. 1991, 1060, 175–188. [Google Scholar] [CrossRef]
- Van Leeuwen, P.; Heimann, C.; van Gorkom, H. Absorbance difference spectra of the S-state transitions in Photosystem II core particles. Photosynth. Res. 1993, 38, 323–330. [Google Scholar] [CrossRef]
- Elizarova, G.L.; Zhidomirov, G.M.; Parmon, V.N. Hydroxides of transition metals as artificial catalysts for oxidation of water to dioxygen. Catal. Today 2000, 58, 71–88. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and soft acids and bases, HSAB, part 1: Fundamental principles. J. Chem. Educ. 1968, 45, 581. [Google Scholar] [CrossRef]
- Gans, P.; Sabatini, A.; Vacca, A. Hypspec 2008 Software; Protonic Software: Leeds, UK; Florence, Italy, 2008. [Google Scholar]
- Alderighi, L.; Gans, P.; Ienco, A.; Peters, D.; Sabatini, A.; Vacca, A. Hyperquad simulation and speciation (HySS): A utility program for the investigation of equilibria involving soluble and partially soluble species. Coord. Chem. Rev. 1999, 184, 311–318. [Google Scholar] [CrossRef]







| Solution composition | [TL] range from 0.054 to 0.342 and 0.030 to 0.600 mM [TM] constant at 0.16 and 0.3 mM | ||
| Ionic strength, electrolyte | Not used | ||
| pH range | Not used | ||
| Experimental method | Spectrophotometric titration | ||
| Temperature | 20 °C | ||
| Total number of data points | Mn complexation: 37 solution spectra | ||
| Method of calculation | HypSpec | ||
| Species | Equilibrium | Log β | σ |
| [Mn(en)]2+ [Mn(en)2]2+ | Mn2++ en ⇄ [Mn(en)]2+ Mn2++2 en ⇄ [Mn(en)2]2+ | log β110 = 3.98 ± 0.029 log β120 = 7.51 ± 0.021 | 0.003 |
| Solution composition | [TL] range from 0.014 to 0.288 and 0.028 to 0.576 mM [TM] constant at 0.144 and 0.288 mM | ||
| Ionic strength, electrolyte | Not used | ||
| pH range | Not used | ||
| Experimental method | Spectrophotometric titration | ||
| Temperature | 20 °C | ||
| Total number of data points | Mn complexation: 40 solution spectra | ||
| Method of calculation | HypSpec | ||
| Species | Equilibrium | Log β | σ |
| [Mn(pn)]2+ [Mn(pn)2]2+ | Mn2+ + pn⇄ [Mn(pn)]2+ Mn2++2 pn⇄ [Mn(pn)2]2+ | log β110 = 5.08 ± 0.033 log β120 = 8.66 ± 0.023 | 0.004 |
| Solution composition | [TL] range from 0.016 to 0.320 and 0.032 to 0.576 mM [TM] constant at 0.160 and 0.320 mM | ||
| Ionic strength, electrolyte | Not used | ||
| pH range | Not used | ||
| Experimental method | Spectrophotometric titration | ||
| Temperature | 20 °C | ||
| Total number of data points | Mn complexation: 38 solution spectra | ||
| Method of calculation | HypSpec | ||
| Species | Equilibrium | Log β | σ |
| [Mn(bn)]2+ [Mn(bn)2]2+ | Mn2+ + bn⇄ [Mn(bn)]2+ Mn2++2 bn⇄ [Mn(bn)2]2+ | log β110 = 4.36 ± 0.044 log β120 = 8.46 ± 0.020 | 0.007 |
| Method | Solvent | Ionic Strength | T(K) | Logβ110 | Logβ120 | Logβ130 | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Cal. | Buffer NH4Cl-NH3 | 0.0510 N AgNO3 | - | 2.76 | 4.87 | 5.76 | [26] | |
| Glass Electrode | Methanol/Water (%) | 0 | 0.1 M KNO3 | 298 | 2.85 | 4.75 | - | [28] |
| 55 | 3.03 | 5.11 | - | |||||
| 60 | 3.07 | 5.17 | - | |||||
| 65 | 3.11 | 5.26 | - | |||||
| 70 | 3.15 | 5.33 | - | |||||
| 75 | 3.23 | 5.51 | - | |||||
| 80 | 3.26 | 5.57 | - | |||||
| Dioxane/Water (%) | 0 | 0.1 M NaClO4 | 298 | 2.79 | 4.69 | - | ||
| 20 | 2.93 | 4.94 | - | |||||
| 40 | 3.16 | 5.36 | - | |||||
| 50 | 3.33 | 5.73 | - | |||||
| 60 | 3.57 | 6.18 | - | |||||
| 70 | 3.90 | 6.85 | 8.55 | |||||
| 75 | 4.06 | 7.19 | 8.99 | |||||
| 80 | 4.27 | 7.56 | 9.66 | |||||
| Acetonitrile/Water (%) | 20 | 0.1 M of KNO3 | 298 | 2.98 | - | - | ||
| 40 | 3.29 | 5.59 | - | |||||
| 50 | 3.44 | 5.94 | - | |||||
| 60 | 3.65 | 6.35 | - | |||||
| 70 | 3.90 | 6.80 | - | |||||
| 75 | 4.10 | 7.20 | - | |||||
| 80 | 4.40 | 8.20 | - | |||||
| DMF */Water | 20 | 0.1 M of KNO3 | 298 | 2.98 | - | - | ||
| 40 | 3.14 | 5.40 | - | |||||
| 50 | 3.26 | 5.60 | 7.00 | |||||
| 60 | 3.40 | 5.87 | 7.45 | |||||
| 70 | 3.59 | 6.25 | 7.83 | |||||
| 75 | 3.71 | 6.47 | 8.28 | |||||
| 80 | 3.95 | 6.84 | 9.01 | |||||
| DMSO * | 0.1 M of KClO4 | 25 | 3.7 | 6.9 | 10.1 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segoviano-Garfias, J.J.N.; Zanor, G.A.; Ávila-Ramos, F. Solution Equilibria Formation of Manganese(II) Complexes with Ethylenediamine, 1,3-Propanediamine and 1,4-ButanediaMine in Methanol. Molbank 2022, 2022, M1367. https://doi.org/10.3390/M1367
Segoviano-Garfias JJN, Zanor GA, Ávila-Ramos F. Solution Equilibria Formation of Manganese(II) Complexes with Ethylenediamine, 1,3-Propanediamine and 1,4-ButanediaMine in Methanol. Molbank. 2022; 2022(2):M1367. https://doi.org/10.3390/M1367
Chicago/Turabian StyleSegoviano-Garfias, José J. N., Gabriela A. Zanor, and Fidel Ávila-Ramos. 2022. "Solution Equilibria Formation of Manganese(II) Complexes with Ethylenediamine, 1,3-Propanediamine and 1,4-ButanediaMine in Methanol" Molbank 2022, no. 2: M1367. https://doi.org/10.3390/M1367