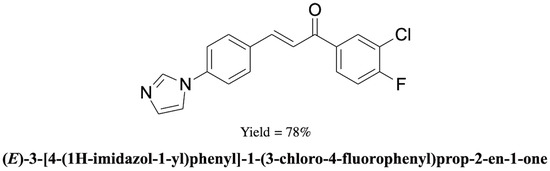
(E)-3-[4-(1H-Imidazol-1-yl)phenyl]-1-(3-chloro-4-fluorophenyl)prop-2-en-1-one
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Dagenais, T.R.T.; Keller, N.P. Pathogenesis of Aspergillus fumigatus in Invasive Aspergillosis. Clin. Microbiol. Rev. 2009, 22, 447–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazaz, R.; Dening, D.W. Aspergillosis: Cause, types and treatment. Pharm. J. 2019, 303, 7927. [Google Scholar]
- Waldeck, F.; Boroli, F.; Suh, N.; Wendel Garcia, P.D.; Flury, D.; Notter, J.; Iten, A.; Kaiser, L.; Schrenzel, J.; Boggian, K.; et al. Influenza-Associated Aspergillosis in Critically-Ill Patients A Retrospective Bicentric Cohort Study. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1915–1923. [Google Scholar] [CrossRef]
- Marr, K.A.; Platt, A.; Tornheim, J.A.; Zhang, S.X.; Datta, K.; Cardozo, C.; Garcia-Vidal, C. Aspergillosis Complicating Severe Coronavirus Disease. Emerg. Infect. Dis. 2021, 27, 18–25. [Google Scholar] [CrossRef]
- Latgé, J.P.; Chamilos, G. Aspergillus fumigatus and Aspergillosis in 2019. Clin. Microbiol. Rev. 2019, 33, e00140-18. [Google Scholar] [CrossRef] [PubMed]
- Tischler, B.Y.; Hohl, T.M. Menacing Mold: Recent Advances in Aspergillus Pathogenesis and Host Defense. J. Mol. Biol. 2019, 431, 4229–4246. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Quispe, C.; Chamkhi, I.; El Omari, N.; Balabib, A.; Sharifi-Rad, J.; Bouyahya, A.; Akram, M.; Iqbal, M.; Docea, A.O.; et al. Pharmacological Properties of Chalcones: A Review of Preclinical Including Molecular Mechanisms and Clinical Evidence. Front. Pharmacol. 2020, 11, 592654. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Joshi, S.; Singh, D. Imidazole: Having Versatile Biological Activities. J. Chem. 2013, 2013, 329412. [Google Scholar] [CrossRef]
- Gupta, D.; Jain, D.K. Chalcone derivatives as potential antifungal agents: Synthesis, and antifungal activity. J. Adv. Pharm. Technol. Res. 2015, 6, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zhou, M.; Wang, W.; Sun, X.; Yarden, O.; Li, S. Abnormal Ergosterol Biosynthesis Activates Transcriptional Responses to Antifungal Azoles. Front. Microbiol. 2018, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Bailey, N.; Atanes, A.; Ashburn, B.O. (E)-3-(4-chlorophenyl)-1-(2-fluoro-4-methoxyphenyl)-2-propen-1-one. Molbank 2021, 2021, M1184. [Google Scholar] [CrossRef]
- Amato-Ocampo, J.; Carrillo, R.; Kae, H.; Ashburn, B.O. Synthesis and Antimicrobial Evaluation of a Series of Chlorinated Chalcone Derivatives. IJPPR Hum. 2018, 13, 112–119. [Google Scholar]
- Bailey, N.; Ashburn, B.O. (E)-3-[4-(1H-imidazol-1-yl)phenyl]-1-(4-methylphenyl)prop-2-en-1-one. Molbank 2021, 2021, M1269. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takaki, R.; Ashburn, B.O. (E)-3-[4-(1H-Imidazol-1-yl)phenyl]-1-(3-chloro-4-fluorophenyl)prop-2-en-1-one. Molbank 2022, 2022, M1375. https://doi.org/10.3390/M1375
Takaki R, Ashburn BO. (E)-3-[4-(1H-Imidazol-1-yl)phenyl]-1-(3-chloro-4-fluorophenyl)prop-2-en-1-one. Molbank. 2022; 2022(2):M1375. https://doi.org/10.3390/M1375
Chicago/Turabian StyleTakaki, Reina, and Bradley O. Ashburn. 2022. "(E)-3-[4-(1H-Imidazol-1-yl)phenyl]-1-(3-chloro-4-fluorophenyl)prop-2-en-1-one" Molbank 2022, no. 2: M1375. https://doi.org/10.3390/M1375