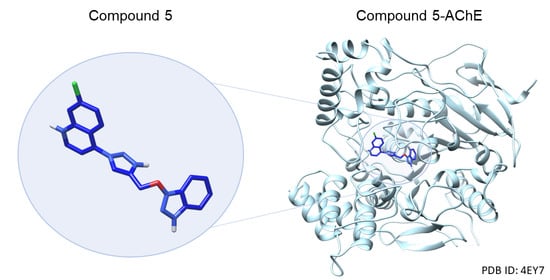
4-(4-(((1H-Benzo[d][1,2,3]triazol-1-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)-7-chloroquinoline
Abstract
:1. Introduction
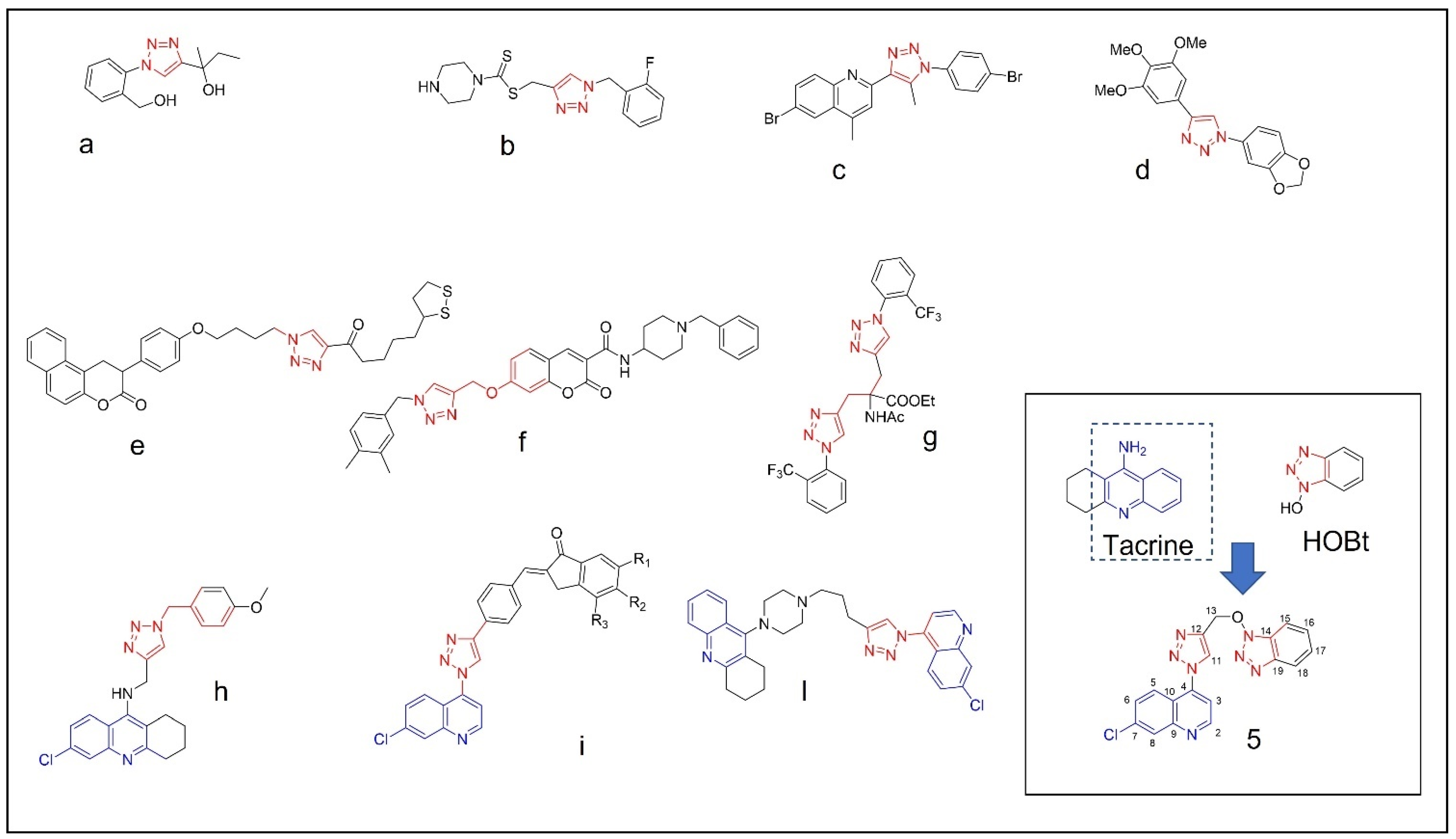
2. Results and Discussion
3. Materials and Methods
3.1. Chemistry
3.1.1. Synthesis of 4-Azido-7-chloro-quinoline (2)
3.1.2. Synthesis of 1-(Prop-2-yn-1-yloxy)-1H-benzo[d][1,2,3]triazole (4)
3.1.3. Synthesis of 4-(4-(((1H-Benzo[d][1,2,3]triazol-1-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)-7-chloroquinolin (5)
3.2. Computational Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Palmer, A.M. Neuroprotective Therapeutics for Alzheimer’s Disease: Progress and Prospects. Trends Pharmacol. Sci. 2011, 32, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; LeVine, H. Alzheimer’s Disease and the Amyloid-β Peptide. JAD 2010, 19, 311–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colovic, M.B.; Krstic, D.Z.; Lazarevic-Pasti, T.D.; Bondzic, A.M.; Vasic, V.M. Acetylcholinesterase Inhibitors: Pharmacology and Toxicology. CN 2013, 11, 315–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, C.; Yadav, D.K.; Subedi, L.; Venkatesan, R.; Venkanna, A.; Afzal, S.; Lee, E.; Yoo, J.; Ji, E.; Kim, S.Y.; et al. Identification of Novel Acetylcholinesterase Inhibitors Designed by Pharmacophore-Based Virtual Screening, Molecular Docking and Bioassay. Sci. Rep. 2018, 8, 14921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makarian, M.; Gonzalez, M.; Salvador, S.M.; Lorzadeh, S.; Hudson, P.K.; Pecic, S. Synthesis, Kinetic Evaluation and Molecular Docking Studies of Donepezil-Based Acetylcholinesterase Inhibitors. J. Mol. Struct. 2022, 1247, 131425. [Google Scholar] [CrossRef]
- Silva, M.A.; Kiametis, A.S.; Treptow, W. Donepezil Inhibits Acetylcholinesterase via Multiple Binding Modes at Room Temperature. J. Chem. Inf. Model. 2020, 60, 3463–3471. [Google Scholar] [CrossRef]
- Shega, J.W.; Ellner, L.; Lau, D.T.; Maxwell, T.L. Cholinesterase Inhibitor and N-Methyl-D-Aspartic Acid Receptor Antagonist Use in Older Adults with End-Stage Dementia: A Survey of Hospice Medical Directors. J. Palliat. Med. 2009, 12, 779–783. [Google Scholar] [CrossRef]
- Zanforlin, E.; Zagotto, G.; Ribaudo, G. An Overview of New Possible Treatments of Alzheimer’s Disease, Based on Natural Products and Semi-Synthetic Compounds. CMC 2017, 24, 3749–3773. [Google Scholar] [CrossRef]
- Ribaudo, G.; Ongaro, A.; Zagotto, G.; Memo, M.; Gianoncelli, A. Therapeutic Potential of Phosphodiesterase Inhibitors against Neurodegeneration: The Perspective of the Medicinal Chemist. ACS Chem. Neurosci. 2020, 11, 1726–1739. [Google Scholar] [CrossRef]
- Ribaudo, G.; Memo, M.; Gianoncelli, A. A Perspective on Natural and Nature-Inspired Small Molecules Targeting Phosphodiesterase 9 (PDE9): Chances and Challenges against Neurodegeneration. Pharmaceuticals 2021, 14, 58. [Google Scholar] [CrossRef]
- Cheung, J.; Rudolph, M.J.; Burshteyn, F.; Cassidy, M.S.; Gary, E.N.; Love, J.; Franklin, M.C.; Height, J.J. Structures of Human Acetylcholinesterase in Complex with Pharmacologically Important Ligands. J. Med. Chem. 2012, 55, 10282–10286. [Google Scholar] [CrossRef] [PubMed]
- Dvir, H.; Silman, I.; Harel, M.; Rosenberry, T.L.; Sussman, J.L. Acetylcholinesterase: From 3D Structure to Function. Chem.-Biol. Interact. 2010, 187, 10–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angajala, K.K.; Vianala, S.; Macha, R.; Raghavender, M.; Thupurani, M.K.; Pathi, P.J. Synthesis, Anti-Inflammatory, Bactericidal Activities and Docking Studies of Novel 1,2,3-Triazoles Derived from Ibuprofen Using Click Chemistry. SpringerPlus 2016, 5, 423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.; Zhao, S.-J.; Liu, Y. 1,2,3-Triazole-Containing Hybrids as Potential Anticancer Agents: Current Developments, Action Mechanisms and Structure-Activity Relationships. Eur. J. Med. Chem. 2019, 183, 111700. [Google Scholar] [CrossRef]
- El Malah, T.; Nour, H.F.; Satti, A.A.E.; Hemdan, B.A.; El-Sayed, W.A. Design, Synthesis, and Antimicrobial Activities of 1,2,3-Triazole Glycoside Clickamers. Molecules 2020, 25, 790. [Google Scholar] [CrossRef] [Green Version]
- Ghiano, D.G.; de la Iglesia, A.; Liu, N.; Tonge, P.J.; Morbidoni, H.R.; Labadie, G.R. Antitubercular Activity of 1,2,3-Triazolyl Fatty Acid Derivatives. Eur. J. Med. Chem. 2017, 125, 842–852. [Google Scholar] [CrossRef] [Green Version]
- Ribaudo, G.; Coghi, P.; Zanforlin, E.; Law, B.Y.K.; Wu, Y.Y.J.; Han, Y.; Qiu, A.C.; Qu, Y.Q.; Zagotto, G.; Wong, V.K.W. Semi-Synthetic Isoflavones as BACE-1 Inhibitors against Alzheimer’s Disease. Bioorg. Chem. 2019, 87, 474–483. [Google Scholar] [CrossRef]
- Jalili-Baleh, L.; Nadri, H.; Forootanfar, H.; Samzadeh-Kermani, A.; Küçükkılınç, T.T.; Ayazgok, B.; Rahimifard, M.; Baeeri, M.; Doostmohammadi, M.; Firoozpour, L.; et al. Novel 3-Phenylcoumarin–Lipoic Acid Conjugates as Multi-Functional Agents for Potential Treatment of Alzheimer’s Disease. Bioorg. Chem. 2018, 79, 223–234. [Google Scholar] [CrossRef]
- Rastegari, A.; Nadri, H.; Mahdavi, M.; Moradi, A.; Mirfazli, S.S.; Edraki, N.; Moghadam, F.H.; Larijani, B.; Akbarzadeh, T.; Saeedi, M. Design, Synthesis and Anti-Alzheimer’s Activity of Novel 1,2,3-Triazole-Chromenone Carboxamide Derivatives. Bioorg. Chem. 2019, 83, 391–401. [Google Scholar] [CrossRef]
- Kaur, A.; Mann, S.; Kaur, A.; Priyadarshi, N.; Goyal, B.; Singhal, N.K.; Goyal, D. Multi-Target-Directed Triazole Derivatives as Promising Agents for the Treatment of Alzheimer’s Disease. Bioorg. Chem. 2019, 87, 572–584. [Google Scholar] [CrossRef]
- Najafi, Z.; Mahdavi, M.; Saeedi, M.; Karimpour-Razkenari, E.; Asatouri, R.; Vafadarnejad, F.; Moghadam, F.H.; Khanavi, M.; Sharifzadeh, M.; Akbarzadeh, T. Novel Tacrine-1,2,3-Triazole Hybrids: In Vitro, in Vivo Biological Evaluation and Docking Study of Cholinesterase Inhibitors. Eur. J. Med. Chem. 2017, 125, 1200–1212. [Google Scholar] [CrossRef] [PubMed]
- Mantoani, S.; Chierrito, T.; Vilela, A.; Cardoso, C.; Martínez, A.; Carvalho, I. Novel Triazole-Quinoline Derivatives as Selective Dual Binding Site Acetylcholinesterase Inhibitors. Molecules 2016, 21, 193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, G.; Gao, Y.; Kang, D.; Huang, B.; Huo, Z.; Liu, H.; Poongavanam, V.; Zhan, P.; Liu, X. Design, Synthesis and Biological Evaluation of Tacrine-1,2,3-Triazole Derivatives as Potent Cholinesterase Inhibitors. Med. Chem. Commun. 2018, 9, 149–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coghi, P.; Ng, J.P.L.; Nasim, A.A.; Wong, V.K.W. N-[7-Chloro-4-[4-(Phenoxymethyl)-1H-1,2,3-Triazol-1-Yl]Quinoline]-Acetamide. Molbank 2021, 2021, M1213. [Google Scholar] [CrossRef]
- Feldman, A.K.; Colasson, B.; Fokin, V.V. One-Pot Synthesis of 1,4-Disubstituted 1,2,3-Triazoles from In Situ Generated Azides. Org. Lett. 2004, 6, 3897–3899. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, S.; Arora, S.; Attri, S.; Kaur, P.; Kaur Gulati, H.; Bhagat, K.; Kumar, N.; Singh, H.; Vir Singh, J.; et al. New Coumarin-Benzotriazole Based Hybrid Molecules as Inhibitors of Acetylcholinesterase and Amyloid Aggregation. Bioorg. Med. Chem. Lett. 2020, 30, 127477. [Google Scholar] [CrossRef]
- Adasme, M.F.; Linnemann, K.L.; Bolz, S.N.; Kaiser, F.; Salentin, S.; Haupt, V.J.; Schroeder, M. PLIP 2021: Expanding the Scope of the Protein–Ligand Interaction Profiler to DNA and RNA. Nucleic Acids Res. 2021, 49, W530–W534. [Google Scholar] [CrossRef]
- Pourshojaei, Y.; Abiri, A.; Eskandari, K.; Haghighijoo, Z.; Edraki, N.; Asadipour, A. Phenoxyethyl Piperidine/Morpholine Derivatives as PAS and CAS Inhibitors of Cholinesterases: Insights for Future Drug Design. Sci. Rep. 2019, 9, 19855. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [Green Version]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Zoete, V. A BOILED-Egg to Predict Gastrointestinal Absorption and Brain Penetration of Small Molecules. ChemMedChem 2016, 11, 1117–1121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castelli, R.; Giacomini, A.; Anselmi, M.; Bozza, N.; Vacondio, F.; Rivara, S.; Matarazzo, S.; Presta, M.; Mor, M.; Ronca, R. Synthesis, Structural Elucidation, and Biological Evaluation of NSC12, an Orally Available Fibroblast Growth Factor (FGF) Ligand Trap for the Treatment of FGF-Dependent Lung Tumors. J. Med. Chem. 2016, 59, 4651–4663. [Google Scholar] [CrossRef] [Green Version]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera? A Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dallakyan, S.; Olson, A.J. Small-Molecule Library Screening by Docking with PyRx. In Chemical Biology; Methods in Molecular Biology; Hempel, J.E., Williams, C.H., Hong, C.C., Eds.; Springer: New York, NY, USA, 2015; Volume 1263, pp. 243–250. ISBN 978-1-4939-2268-0. [Google Scholar]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2009, 31, 455–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fai, L.K.; Anyanwu, M.; Ai, J.; Xie, Y.; Gianoncelli, A.; Ribaudo, G.; Coghi, P. 4-(4-(((1H-Benzo[d][1,2,3]triazol-1-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)-7-chloroquinoline. Molbank 2022, 2022, M1404. https://doi.org/10.3390/M1404
Fai LK, Anyanwu M, Ai J, Xie Y, Gianoncelli A, Ribaudo G, Coghi P. 4-(4-(((1H-Benzo[d][1,2,3]triazol-1-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)-7-chloroquinoline. Molbank. 2022; 2022(3):M1404. https://doi.org/10.3390/M1404
Chicago/Turabian StyleFai, Leong Ka, Margrate Anyanwu, Jiang Ai, Yuhan Xie, Alessandra Gianoncelli, Giovanni Ribaudo, and Paolo Coghi. 2022. "4-(4-(((1H-Benzo[d][1,2,3]triazol-1-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)-7-chloroquinoline" Molbank 2022, no. 3: M1404. https://doi.org/10.3390/M1404