(S)-N1,N3-Dibenzyl-1-cyclohexyl-N1,N3-bis((R)-1-phenylethyl)propane-1,3-diamine
Abstract
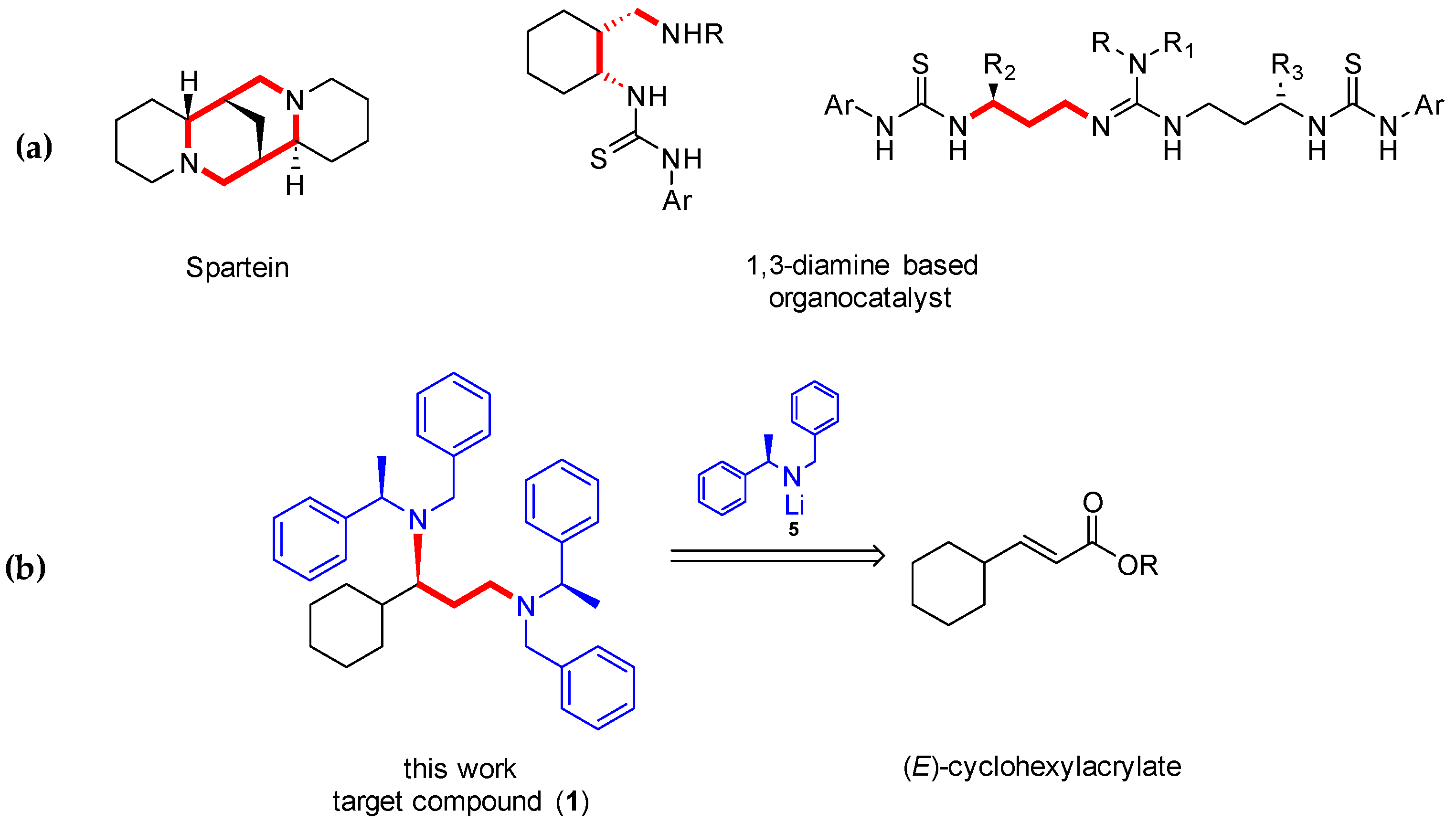
:1. Introduction
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hidalgo, M.; Asmat Marrufo, P.; Lezama Asencio, P.; Ramos, C.; Chimoy Tuñoque, C.A.; Zolla, G. Evaluation of in vitro susceptibility to sparteine in four strains of Mycobacterium tuberculosis. Rev. Peru. Med. Exp. Salud Pública 2022, 39, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Kassenbrock, A.; Li, B.X.; Xiao, X. Discovery of a potent anti-tumor agent through regioselective mono-N-acylation of 7 H-pyrrolo [3, 2-f] quinazoline-1, 3-diamine. MedChemComm 2013, 4, 1275–1282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pugsley, M.K.; Saint, D.A.; Hayes, E.; Berlin, K.D.; Walker, M.J. The cardiac electrophysiological effects of sparteine and its analogue BRB-I-28 in the rat. Eur. J. Pharmacol. 1995, 294, 319–327. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, Q.; Lee, C.S.; Wang, J. Enantio-and Regioselective Construction of 1, 4-Diamines via Cascade Hydroamination of Methylene Cyclopropanes. Angew. Chem. Int. Ed. 2022, 61, e202202160. [Google Scholar] [CrossRef]
- Gawali, V.S.; Simeonov, S.; Drescher, M.; Knott, T.; Scheel, O.; Kudolo, J.; Kählig, H.; Hochenegg, U.; Roller, A.; Todt, H. C2-Modified Sparteine Derivatives Are a New Class of Potentially Long-Acting Sodium Channel Blockers. ChemMedChem 2017, 12, 1819–1822. [Google Scholar] [CrossRef] [PubMed]
- Gavilan, J.; Mennickent, D.; Ramirez-Molina, O.; Trivino, S.; Perez, C.; Silva-Grecchi, T.; Godoy, P.A.; Becerra, J.; Aguayo, L.G.; Moraga-Cid, G. 17 Oxo Sparteine and Lupanine, Obtained from Cytisus scoparius, Exert a Neuroprotection against Soluble Oligomers of Amyloid-β Toxicity by Nicotinic Acetylcholine Receptors. J. Alzheimer’s Dis. 2019, 67, 343–356. [Google Scholar] [CrossRef]
- Kodama, K.; Sugawara, K.; Hirose, T. Synthesis of Chiral 1, 3-Diamines Derived from cis-2-Benzamidocyclohexanecarboxylic Acid and Their Application in the Cu-Catalyzed Enantioselective Henry Reaction. Chem. Eur. J. 2011, 17, 13584–13592. [Google Scholar] [CrossRef]
- Kizirian, J. Chiral tertiary diamines in asymmetric synthesis. Chem. Rev. 2008, 108, 140–205. [Google Scholar] [CrossRef]
- Mayans, E.; Gargallo, A.; Álvarez-Larena, Á.; Illa, O.; Ortuño, R.M. Diastereodivergent Synthesis of Chiral vic-Disubstituted-Cyclobutane Scaffolds: 1, 3-Amino Alcohol and 1, 3-Diamine Derivatives–Preliminary Use in Organocatalysis. Eur. J. Org. Chem. 2013, 2013, 1425–1433. [Google Scholar] [CrossRef]
- Csillag, K.; Szakonyi, Z.; Fülöp, F. Stereoselective syntheses of pinane-based 1, 3-diamines and their application as chiral ligands in the enantioselective addition of diethylzinc to benzaldehyde. Tetrahedron Asymmetry 2013, 24, 553–561. [Google Scholar] [CrossRef]
- Ji, X.; Huang, H. Synthetic methods for 1, 3-diamines. Org. Biomol. Chem. 2016, 14, 10557–10566. [Google Scholar] [CrossRef]
- Li, K.; Weber, A.E.; Tseng, L.; Malcolmson, S.J. Diastereoselective and enantiospecific synthesis of 1, 3-diamines via 2-azaallyl anion benzylic ring-opening of aziridines. Org. Lett. 2017, 19, 4239–4242. [Google Scholar] [CrossRef] [PubMed]
- Rossolini, T.; Leitch, J.A.; Grainger, R.; Dixon, D.J. Photocatalytic three-component umpolung synthesis of 1, 3-diamines. Org. Lett. 2018, 20, 6794–6798. [Google Scholar] [CrossRef] [PubMed]
- Ričko, S.; Svete, J.; Štefane, B.; Perdih, A.; Golobič, A.; Meden, A.; Grošelj, U. 1, 3-Diamine-Derived Bifunctional Organocatalyst Prepared from Camphor. Adv. Synth. Catal. 2016, 358, 3786–3796. [Google Scholar] [CrossRef]
- Davies, S.G.; Smith, A.D.; Price, P.D. The conjugate addition of enantiomerically pure lithium amides as homochiral ammonia equivalents: Scope, limitations and synthetic applications. Tetrahedron Asymmetry 2005, 16, 2833–2891. [Google Scholar] [CrossRef]
- Davies, S.G.; Fletcher, A.M.; Roberts, P.M.; Thomson, J.E. The conjugate addition of enantiomerically pure lithium amides as chiral ammonia equivalents part II: 2005–2011. Tetrahedron Asymmetry 2012, 23, 1111–1153. [Google Scholar] [CrossRef]
- Davies, S.G.; Fletcher, A.M.; Roberts, P.M.; Thomson, J.E. The conjugate addition of enantiomerically pure lithium amides as chiral ammonia equivalents part III: 2012–2017. Tetrahedron Asymmetry 2017, 28, 1842–1868. [Google Scholar] [CrossRef]
- Garrido, N.M.; Nieto, C.T.; Diez, D. Enantioselective synthesis of a (1R, 5R, 9R)-2-azabicyclo [3.3.1] nonane-9-carboxylic acid with an embedded morphan motif: A multipurpose product. Synlett 2013, 24, 169–172. [Google Scholar] [CrossRef]
- Nieto, C.T.; Gonzalez-Nunez, V.; Rodríguez, R.E.; Diez, D.; Garrido, N.M. Design, synthesis, pharmacological evaluation and molecular dynamics of β-amino acids morphan-derivatives as novel ligands for opioid receptors. Eur. J. Med. Chem. 2015, 101, 150–162. [Google Scholar] [CrossRef]
- Salgado, M.M.; Manchado, A.; Nieto, C.T.; Díez, D.; Garrido, N.M. Synthesis and Modeling of Ezetimibe Analogues. Molecules 2021, 26, 3107. [Google Scholar] [CrossRef]
- Urones, J.G.; Garrido, N.M.; Díez, D.; Dominguez, S.H.; Davies, S.G. Asymmetric synthesis of (R)-and (S)-methyl (2-methoxy-carbonylcyclopent-2-enyl) acetate and (R)-and (S)-2-(2-hydroxy-methyl-cyclopent-2-enyl) ethanol. Tetrahedron Asymmetry 1997, 8, 2683–2685. [Google Scholar] [CrossRef]
- Davies, S.G.; Walters, I.A. Asymmetric synthesis of anti-α-alkyl-β-amino Acids. J. Chem. Soc. Perkin Trans. 1 1994, 1129–1139. [Google Scholar] [CrossRef]
- Davies, S.G.; Ichihara, O. Asymmetric synthesis of R-β-amino butanoic acid and S-β-tyrosine: Homochiral lithium amide equivalents for Michael additions to α, β-unsaturated esters. Tetrahedron Asymmetry 1991, 2, 183–186. [Google Scholar] [CrossRef]
- Koperniku, A.; Schafer, L.L. Zirconium catalyzed hydroaminoalkylation for the synthesis of α-Arylated amines and N-Heterocycles. Chem. Eur. J. 2021, 27, 6334–6339. [Google Scholar] [CrossRef]
- Das, M.; Manvar, A.; Fox, I.; Roberts, D.J.; O’Shea, D.F. Bu4N-controlled addition and olefination with ethyl 2-(trimethylsilyl) acetate via silicon activation. Synlett 2017, 28, 2401–2406. [Google Scholar]
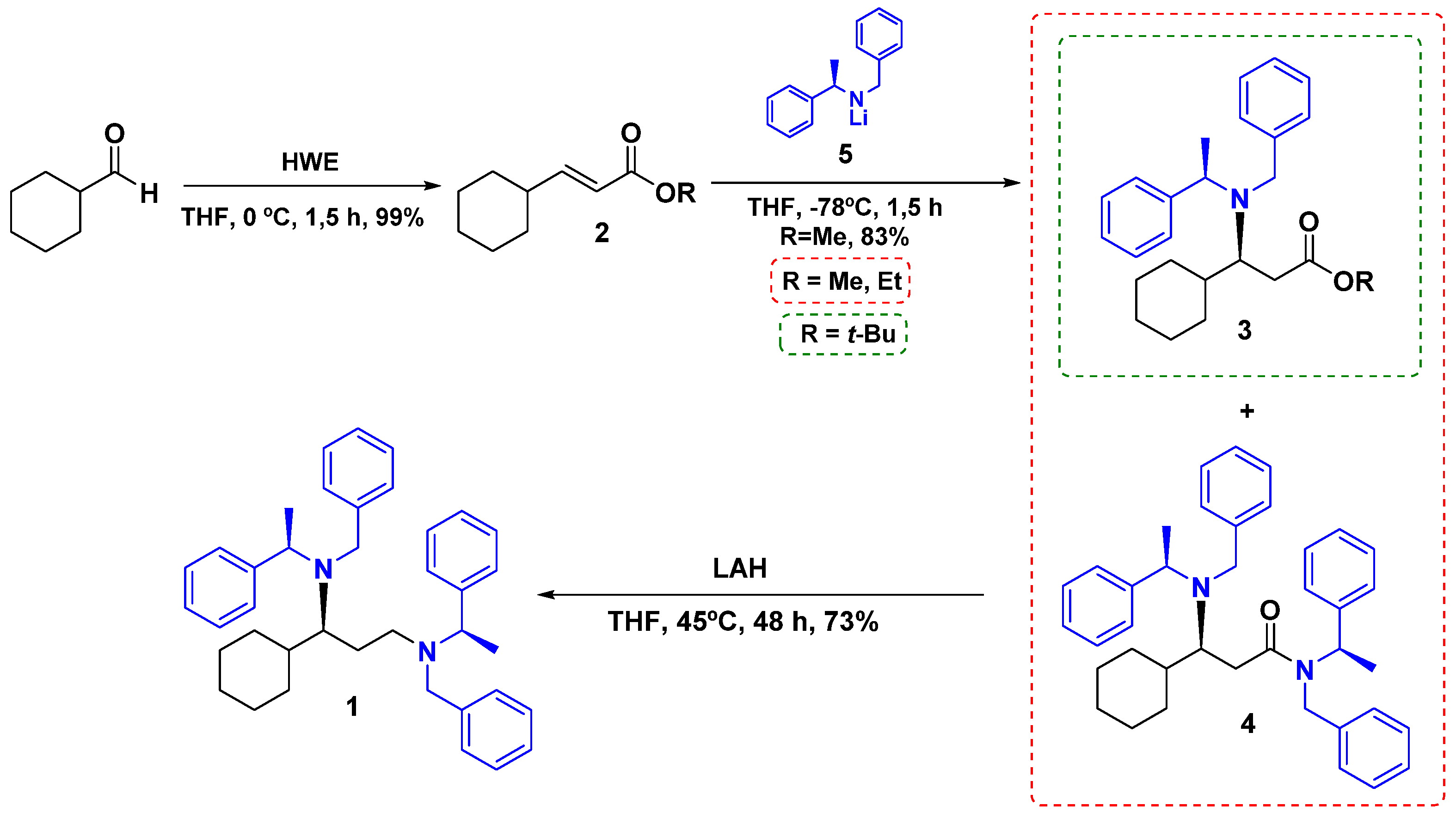
| Entry | R | Eq (5) | Yield (3)(%) | Yield (4)(%) |
|---|---|---|---|---|
| 1 | Tert-butyl | 2.2 | 98 | - |
| 2 | Ethyl | 2.2 | 37 | 54 |
| 3 | Methyl | 2.2 | 20 | 72 |
| 4 | Methyl | 4.4 | - | 83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belda, L.; García-González, Á.; Manchado, A.; Nieto, C.T.; Garrido, N.M. (S)-N1,N3-Dibenzyl-1-cyclohexyl-N1,N3-bis((R)-1-phenylethyl)propane-1,3-diamine. Molbank 2023, 2023, M1544. https://doi.org/10.3390/M1544
Belda L, García-González Á, Manchado A, Nieto CT, Garrido NM. (S)-N1,N3-Dibenzyl-1-cyclohexyl-N1,N3-bis((R)-1-phenylethyl)propane-1,3-diamine. Molbank. 2023; 2023(1):M1544. https://doi.org/10.3390/M1544
Chicago/Turabian StyleBelda, Leland, Ángel García-González, Alejandro Manchado, Carlos T. Nieto, and Narciso M. Garrido. 2023. "(S)-N1,N3-Dibenzyl-1-cyclohexyl-N1,N3-bis((R)-1-phenylethyl)propane-1,3-diamine" Molbank 2023, no. 1: M1544. https://doi.org/10.3390/M1544






