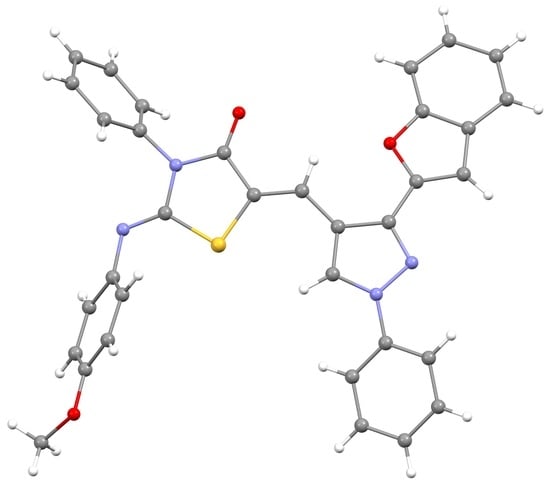
(2Z,5Z)-5-((3-(Benzofuran-2-yl)-1-phenyl-1H-pyrazol-4-yl)methylene)-2-((4-methoxyphenyl)imino)-3-phenylthiazolidin-4-one
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of 3
2.2. IR and NMR Spectroscopy of 3
2.3. Crystal Structure of 3
3. Materials and Methods
3.1. General
3.2. Synthesis of 3
3.3. Crystal Structure Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Rangaswamy, J.; Kumar, H.V.; Harini, S.T.; Naik, N. Functionalized 3-(benzofuran-2-yl)-5-(4-methoxyphenyl)-4,5-dihydro-1H-pyrazole scaffolds: A new class of antimicrobials and antioxidants. Arab. J. Chem. 2017, 10, S2685–S2696. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Zhao, S.; Lv, Z.; Feng, L.; Wang, Y.; Zhang, F.; Bai, L.; Deng, J. Benzofuran derivatives and their anti-tubercular, anti-bacterial activities. Eur. J. Med. Chem. 2019, 162, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.H.; Hu, Y.H.; Yang, J.; Liu, T.; Sun, J.; Wang, X.J. Natural source, bioactivity and synthesis of benzofuran derivatives. RSC Adv. 2019, 9, 27510–27540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbas, A.A.; Dawood, K.M. Anticancer therapeutic potential of benzofuran scaffolds. RSC Adv. 2023, 13, 11096–11120. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, B.F.; Abdel-Aziz, H.A.; Ahmed, E.M. Synthesis and antimicrobial evaluation of 1-(benzofuran-2-yl)-4-nitro-3-arylbutan-1-ones and 3-(benzofuran-2-yl)-4,5-dihydro-5-aryl-1-[4-(aryl)-1,3-thiazol-2-yl]-1H-pyrazoles. Eur. J. Med. Chem. 2009, 44, 2632–2635. [Google Scholar] [CrossRef]
- Abdel-Wahab, B.F.; Abdel-Aziz, H.A.; Ahmed, E.M. Convenient synthesis and antimicrobial activity of new 3-substituted 5-(benzofuran-2-yl)-pyrazole derivatives. Arch. Pharm. 2008, 341, 734–739. [Google Scholar] [CrossRef]
- Abd El-Karim, S.S.; Anwar, M.M.; Mohamed, N.A.; Nasr, T.; Elseginy, S.A. Design, synthesis, biological evaluation and molecular docking studies of novel benzofuran-pyrazole derivatives as anticancer agents. Bioorg. Chem. 2015, 63, 1–12. [Google Scholar] [CrossRef]
- Farhat, J.; Alzyoud, L.; Alwahsh, M.; Al-Omari, B. Structure-activity relationship of benzofuran derivatives with potential anticancer activity. Cancers 2022, 14, 2196. [Google Scholar] [CrossRef]
- Anwar, M.M.; Abd El-Karim, S.S.; Mahmoud, A.H.; Amr, A.E.E.; Al-Omar, M.A. A comparative study of the anticancer activity and PARP-1 inhibiting effect of benzofuran-pyrazole scaffold and its nano-sized particles in human breast cancer cells. Molecules 2019, 24, 2413. [Google Scholar] [CrossRef] [Green Version]
- Mech, D.; Kurowska, A.; Trotsko, N. The Bioactivity of thiazolidin-4-ones: A short review of the most recent studies. Int. J. Mol. Sci. 2021, 22, 11533. [Google Scholar] [CrossRef]
- Tripathi, A.C.; Gupta, S.J.; Fatima, G.N.; Sonar, P.K.; Verma, A.; Saraf, S.K. 4-Thiazolidinones: The advances continue. Eur. J. Med. Chem. 2014, 72, 52–77. [Google Scholar] [CrossRef] [PubMed]
- Hammad, S.G.; El-Gazzar, M.G.; Abutaleb, N.S.; Li, D.; Ramming, I.; Shekhar, A.; Abdel-Halim, M.; Elrazaz, E.Z.; Seleem, M.N.; Bilitewski, U.; et al. Synthesis and antimicrobial evaluation of new halogenated 1,3-thiazolidin-4-ones. Bioorg. Chem. 2020, 95, 103517. [Google Scholar] [CrossRef] [PubMed]
- Revelant, G.; Huber-Villaume, S.; Dunand, S.; Kirsch, G.; Schohn, H.; Hesse, S. Synthesis and biological evaluation of novel 2-heteroarylimino-1,3-thiazolidin-4-ones as potential anti-tumor agents. Eur. J. Med. Chem. 2015, 94, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.S.; Kaur, R.; Bhatia, R.; Kumar, K.; Singh, V.; Shankar, R.; Kaur, R.; Rawal, R.K. Synthetic and medicinal perspective of thiazolidinones: A review. Bioorg. Chem. 2017, 75, 406–423. [Google Scholar] [CrossRef]
- Levshin, I.B.; Simonov, A.Y.; Lavrenov, S.N.; Panov, A.A.; Grammatikova, N.E.; Alexandrov, A.A.; Ghazy, E.S.M.O.; Savin, N.A.; Gorelkin, P.V.; Erofeev, A.S.; et al. Antifungal thiazolidines: Synthesis and biological evaluation of mycosidine congeners. Pharmaceuticals 2022, 15, 563. [Google Scholar] [CrossRef] [PubMed]
- Opletalova, V.; Dolezel, J.; Kunes, J.; Buchta, V.; Vejsova, M.; Kucerova-Chlupacova, M. Synthesis and antifungal screening of 2-{[1-(5-alkyl/arylalkylpyrazin-2-yl)ethylidene]hydrazono}-1,3-thiazolidin-4-ones. Molecules 2016, 21, 1592. [Google Scholar] [CrossRef] [Green Version]
- Bielenica, A.; Sanna, G.; Madeddu, S.; Struga, M.; Jóźwiak, M.; Kozioł, A.E.; Sawczenko, A.; Materek, I.B.; Serra, A.; Giliberti, G. New thiourea and 1,3-thiazolidin-4-one derivatives effective on the HIV-1 virus. Chem. Biol. Drug Des. 2017, 90, 883–891. [Google Scholar] [CrossRef]
- Gökce, H.; Şen, F.; Sert, Y.; Abdel-Wahab, B.F.; Kariuki, B.M.; El-Hiti, G.A. Quantum computational investigation of (E)-1-(4-methoxyphenyl)-5-methyl-N’-(3-phenoxybenzylidene)-1H-1,2,3-triazole-4-carbohydrazide. Molecules 2022, 27, 2193. [Google Scholar] [CrossRef]
- Kariuki, B.M.; Abdel-Wahab, B.F.; Mohamed, H.A.; Bekheit, M.S.; El-Hiti, G.A. Synthesis and characterization of novel 2-(1,2,3-triazol-4-yl)-4,5-dihydro-1H-pyrazol-1-yl)thiazoles and 2-(4,5-dihydro-1H-pyrazol-1-yl)-4-(1H-1,2,3-triazol-4-yl)thiazoles. Molecules 2022, 27, 8904. [Google Scholar] [CrossRef]
- Kariuki, B.M.; Abdel-Wahab, B.F.; Bekheit, M.S.; El-Hiti, G.A. (Z)-2-(1-(5-Methyl-1-(4-nitrophenyl)-1H-1,2,3-triazol-4-yl)ethylidene)-N-phenylhydrazine-1-carbothioamide. Molbank 2022, 2022, M1462. [Google Scholar] [CrossRef]
- Kariuki, B.M.; Abdel-Wahab, B.F.; Bekheit, M.S.; El-Hiti, G.A. Intermolecular interactions of 3,5-bis(4-methoxyphenyl)-4,5-dihydro-1H-pyrazole-1-carbothioamide in a cocrystal with 1,3-bis(4-methoxyphenyl)prop-2-en-1-one and dimethylformamide Solvate. Crystals 2022, 12, 663. [Google Scholar] [CrossRef]
- Abdel-Wahab, B.F.; Mohamed, H.A.; Farahat, A.A.; Kariuki, B.M.; El-Hiti, G.A. Reactivity of 4-bromoacetyl-1,2,3-triazoles towards amines and phenols: Synthesis and antimicrobial activity of novel heterocycles. Heterocycles 2022, 104, 1601–1613. [Google Scholar] [CrossRef]
- Ali, Y.; Alam, M.S.; Hamid, H.; Husain, A.; Dhulap, A.; Hussain, F.; Bano, S.; Kharbanda, C. Molecular modeling and synthesis of some new 2-imino-4-thiazolidinone derivatives with promising TNF-α inhibitory activity. New J. Chem. 2016, 40, 711–723. [Google Scholar] [CrossRef]
- Viveka, S.; Dinesha, D.; Shama, P.; Naveen, S.; Lokanath, N.K.; Nagaraja, G.K. Design, synthesis, anticonvulsant and analgesic studies of new pyrazole analogues: A Knoevenagel reaction approach. RSC Adv. 2015, 5, 94786–94795. [Google Scholar] [CrossRef] [Green Version]
- El-Zahar, M.I.; Abd El-Karim, S.S.; Anwar, M.M. Synthesis and cytotoxicity screening of some novel benzofuranoyl-pyrazole derivatives against liver and cervix carcinoma cell lines. S. Afr. J. Chem. 2008, 62, 189–199. [Google Scholar]
- Kosurkar, U.B.; Dadmal, T.L.; Kumbhare, R.M.; Sridhar, B. An environmentally benign water promoted catalyst free highly efficient, one-pot synthesis of 2-imino-4-thiazolidinone. J. Heterocycl. Chem. 2014, 51, 1522–1527. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef] [Green Version]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Wahab, B.F.; Mohamed, H.A.; Kariuki, B.M.; El-Hiti, G.A. (2Z,5Z)-5-((3-(Benzofuran-2-yl)-1-phenyl-1H-pyrazol-4-yl)methylene)-2-((4-methoxyphenyl)imino)-3-phenylthiazolidin-4-one. Molbank 2023, 2023, M1665. https://doi.org/10.3390/M1665
Abdel-Wahab BF, Mohamed HA, Kariuki BM, El-Hiti GA. (2Z,5Z)-5-((3-(Benzofuran-2-yl)-1-phenyl-1H-pyrazol-4-yl)methylene)-2-((4-methoxyphenyl)imino)-3-phenylthiazolidin-4-one. Molbank. 2023; 2023(2):M1665. https://doi.org/10.3390/M1665
Chicago/Turabian StyleAbdel-Wahab, Bakr F., Hanan A. Mohamed, Benson M. Kariuki, and Gamal A. El-Hiti. 2023. "(2Z,5Z)-5-((3-(Benzofuran-2-yl)-1-phenyl-1H-pyrazol-4-yl)methylene)-2-((4-methoxyphenyl)imino)-3-phenylthiazolidin-4-one" Molbank 2023, no. 2: M1665. https://doi.org/10.3390/M1665