(5Z,9Z)-14-[(3,28-Dioxoolean-12-en-28-yl)oxy]tetradeca-5,9-dienoic Acid with Cytotoxic Activity
Abstract
:1. Introduction
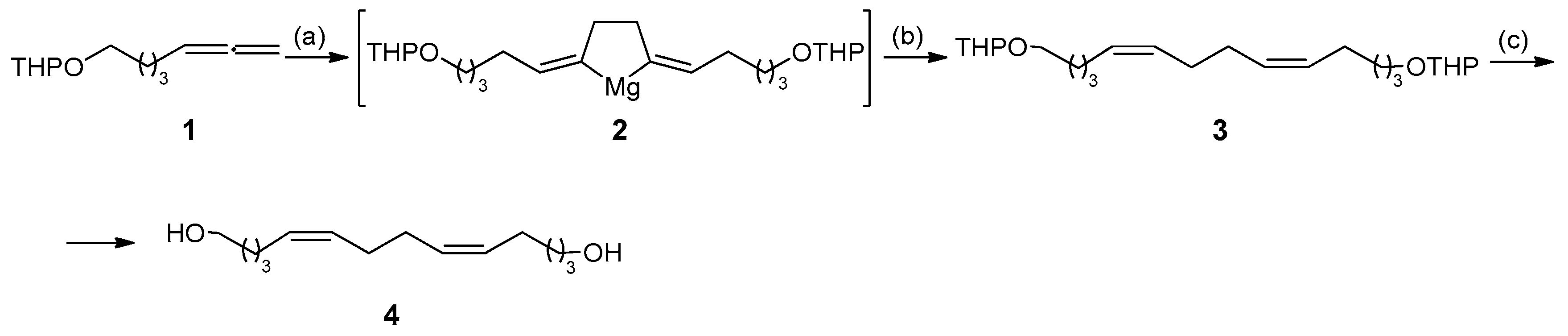
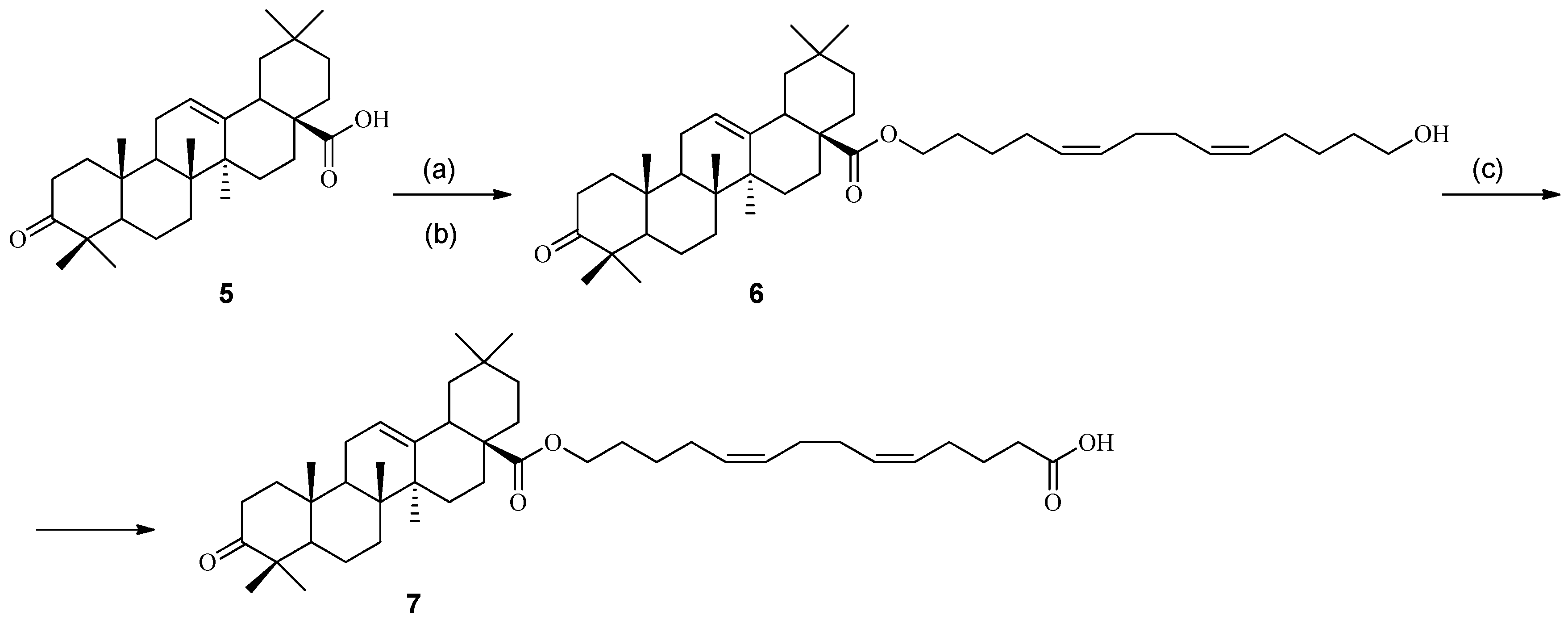
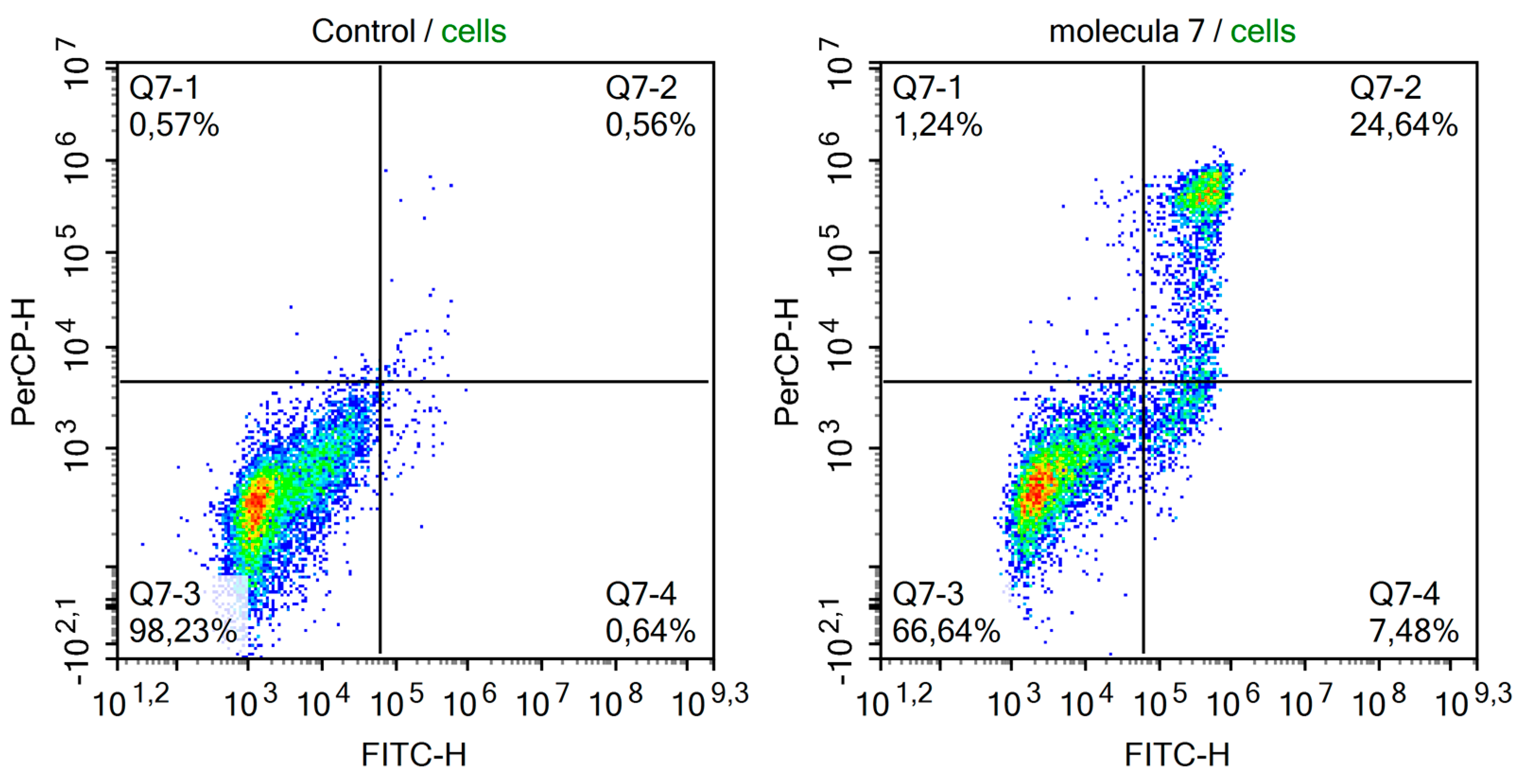
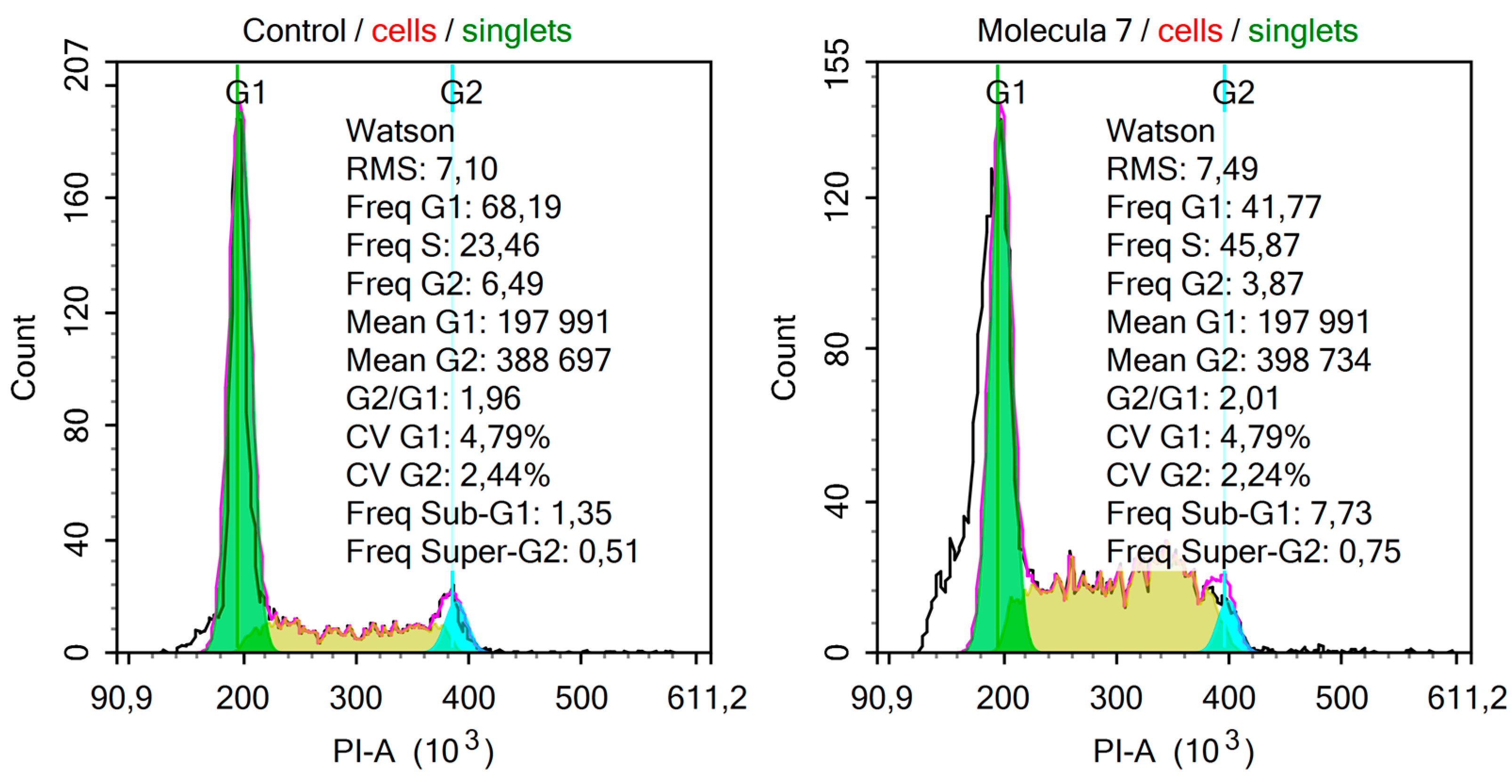
2. Results and Discussion
3. Materials and Methods
3.1. Chemistry
3.2. Biological Screening
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Debas, H.T.; Laxminarayan, R.; Straus, S.E. Complementary and alternative medicine. In Disease Control Priorities in Developing Countries, 2nd ed.; Jamison, D.T., Breman, J.G., Measham, A.R., Alleyne, G., Claeson, M., Evans, D.B., Jha, P., Mills, A., Musgrove, P., Eds.; Oxford University Press: New York, NY, USA, 2006; pp. 1281–1291. ISBN 10 0-8213-6179-1. [Google Scholar]
- Fai, Y.M.; Tao, C.C. A review of presence of oleanolic acid in natural products. Nat. Prod. Med. 2009, 2, 77–290. [Google Scholar]
- Guinda, Á.; Pérez-Camino, M.C.; Lanzón, A. Supplementation of oils with oleanolic acid from the olive leaf (Olea europaea). Eur. J. Lipid Sci. Technol. 2004, 106, 22–26. [Google Scholar] [CrossRef]
- Pollier, J.; Goossens, A. Oleanolic acid. Phytochemistry 2012, 77, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Heinzen, H.; de Vries, J.X.; Moyna, P.; Remberg, G.; Martinez, R.; Tietze, L.F. Mass spectrometry of labelled triterpenoids: Thermospray and electron impact ionization analysis. Phytochem. Anal. 1996, 7, 237–244. [Google Scholar] [CrossRef]
- Wang, X.; Ye, X.L.; Liu, R.; Chen, H.L.; Bai, H.; Liang, X.; Zhang, X.D.; Wang, Z.; Li, W.L.; Hai, C.X. Antioxidant activities of oleanolic acid in vitro: Possible role of Nrf2 and MAP kinases. Chem. Biol. Interact. 2010, 184, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, R.; Zhang, W.; Zhang, X.; Liao, N.; Wang, Z.; Li, W.; Qin, X.; Hai, C. Oleanolic acid improves hepatic insulin resistance via antioxidant, hypolipidemic and anti-inflammatory effects. Mol. Cell. Endocrinol. 2013, 376, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.Y.; Huang, H.Y.; Wu, Y.L. Anticancer and apoptotic activities of oleanolic acid are mediated through cell cycle arrest and disruption of mitochondrial membrane potential in HepG2 human hepatocellular carcinoma cells. Mol. Med. Rep. 2015, 12, 5012–5018. [Google Scholar] [CrossRef]
- Jesus, J.A.; Lago, J.H.G.; Laurenti, M.D.; Yamamoto, E.S.; Passero, L.F.D. Antimicrobial activity of oleanolic and ursolic acids: An update. Evid. Based Complement. Altern. Med. 2015, 2015, 620472. [Google Scholar] [CrossRef]
- Yoo, S.R.; Jeong, S.J.; Lee, N.R.; Shin, H.K.; Seo, C.S. Quantification analysis and In vitro anti-inflammatory effects of 20-hydroxyecdysone, momordin ic, and oleanolic acid from the fructus of Kochia scoparia. Pharmacogn. Mag. 2017, 13, 339–344. [Google Scholar]
- Zhao, H.; Zhou, M.; Duan, L.; Wang, W.; Zhang, J.; Wang, D.; Liang, X. Efficient synthesis and anti-fungal activity of oleanolic acid oxime esters. Molecules 2013, 18, 3615–3629. [Google Scholar] [CrossRef]
- Siddiqui, S.; Siddiqui, B.S.; Adil, Q.; Begum, S. Constituents of Mirabilis jalapa. Fitoterapia 1990, 61, 471. [Google Scholar]
- Yu, F.; Wang, Q.; Zhang, Z.; Peng, Y.; Qiu, Y.; Shi, Y.; Zheng, Y.; Xiao, S.; Wang, H.; Huang, X.; et al. Development of oleanane-type triterpenes as a new class of HCV entry inhibitors. J. Med. Chem. 2013, 56, 4300–4319. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Liby, K.T.; Stephenson, K.K.; Holtzclaw, W.D.; Gao, X.; Suh, N.; Williams, C.; Risingsong, R.; Honda, T.; Gribble, G.W.; et al. Extremely potent triterpenoid inducers of the phase 2 response: Correlations of protection against oxidant and inflammatory stress. Proc. Natl. Acad. Sci. USA 2005, 102, 4584–4589. [Google Scholar] [CrossRef] [PubMed]
- Geetha, T.; Varalakshmi, P.; Latha, R.M. Effect of triterpenes from Crataeva nurvala stem bark on lipid peroxidation in adjuvant induced arthritis in rats. Pharmacol. Res. 1998, 37, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Geetha, T.; Varalakshmi, P. Anticomplement activity of triterpenes from Crataeva nurvala stem bark in adjuvant arthritis in rats. Gen. Pharmacol. Vasc. Syst. 1999, 32, 495–497. [Google Scholar] [CrossRef] [PubMed]
- Geetha, T.; Maralakshmi, P. Effect of lupeol and lupeol linoleate on lysosomal enzymes and collagen in adjuvant-induced arthritis in rats. Mol. Cell. Biochem. 1999, 201, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Geetha, T.; Varalakshmi, P. Anti-inflammatory activity of lupeol and lupeol linoleate in rats. J. Ethnopharmacol. 2001, 76, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Mayaux, J.F.; Bousseau, A.; Pauwels, R.; Huet, T.; Hénin, Y.; Dereu, N.; Evers, M.; Soler, F.; Poujade, C.; De Clercq, E.; et al. Triterpene derivatives that block entry of human immunodeficiency virus type 1 into cells. Proc. Natl. Acad. Sci. USA 1994, 91, 3564–3568. [Google Scholar] [CrossRef]
- Fujioka, T.; Kashiwada, Y.; Kilkuskie, R.E.; Cosentino, L.M.; Ballas, L.M.; Jiang, J.B.; Janzen, W.P.; Chen, I.S.; Lee, K.H. Anti-AIDS agents, 11. Betulinic acid and platanic acid as anti-HIV principles from Syzigium claviflorum, and the anti-HIV activity of structurally related triterpenoids. J. Nat. Prod. 1994, 57, 243–247. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Dzhemileva, L.U.; Dzhemilev, U.M. Natural Compounds with bis-Methylene-Interrupted Z-Double Bonds: Plant Sources, Strategies of Total Synthesis, Biological Activity, and Perspectives. Phytochem. Rev. 2021, 20, 325–342. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Makarov, A.A.; Dzhemileva, L.U.; Ramazanov, I.R.; Makarova, E.K.; Dzhemilev, U.M. Natural Trienoic Acids as Anticancer Agents: First Stereoselective Synthesis, Cell Cycle Analysis, Induction of Apoptosis, Cell Signaling and Mitochondrial Targeting Studies. Cancers 2021, 13, 1808. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M.; Srebnik, M. Natural halogenated fatty acids:their analogues and derivatives. Prog. Lipid Res. 2002, 41, 315–367. [Google Scholar] [CrossRef] [PubMed]
- Carballeira, N.M.; Emiliano, A.; Guzman, A. Facile syntheses for (5Z,9Z)-5,9-hexadecadienoic acid, (5Z,9Z)-5,9-nonadecadienoic acid, and (5Z,9Z)-5,9-eicosadienoic acid through a common synthetic route. Chem. Phys. Lipids 1999, 100, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Carballeira, N.M.; Reyes, E.D.; Sostre, A.; Rodriguez, A.D.; Rodriguez, J.L.; Gonzalez, F.A. Identification of the Novel Antimicrobial Fatty Acid (5Z,9Z)-14-Methyl-5,9-pentadecadienoic Acid in Eunicea succinea. J. Nat. Prod. 1997, 60, 502–504. [Google Scholar] [CrossRef]
- Carballeira, N.M. New advances in fatty acids as antimalarial, antimycobacterial and antifungal agents. Prog. Lipid Res. 2008, 47, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Carballeira, N.M.; Betancourt, J.E.; Orellano, E.A.; Gonzalez, F.A. Total Synthesis and Biological Evaluation of (5Z,9Z)-5,9-Hexadecadienoic Acid, an Inhibitor of Human Topoisomerase I. J. Nat. Prod. 2002, 65, 1715–1718. [Google Scholar] [CrossRef] [PubMed]
- Dzhemilev, U.M.; D’yakonov, V.A.; Tuktarova, R.A.; Dzhemileva, L.U.; Ishmukhametova, S.R.; Yunusbaeva, M.M.; de Meijere, A. Short Route to the Total Synthesis of Natural Muricadienin, and Investigation of Its Cytotoxic Properties. J. Nat. Prod. 2016, 79, 2039–2044. [Google Scholar] [CrossRef]
- Nemoto, T.; Yoshino, G.; Ojika, M.; Sakagam, Y. Amphimic Acids and Related Long-chain Fatty Acids as DNA Topoisomerase I Inhibitors from an Australian Sponge, Amphimedon sp.: Isolation, Structure, Synthesis, and Biological Evaluation. Tetrahedron 1997, 53, 16699–16710. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Dzhemileva, L.U.; Makarov, A.A.; Mulyukova, A.R.; Baev, D.S.; Khusnutdinova, E.K.; Tolstikova, T.G.; Dzhemilev, U.M. nZ,(n+4)Z-Dienoic fatty acid: A new method for the synthesis and inhibitory action on topoisomerase I and II α. Med. Chem. Res. 2016, 25, 30–39. [Google Scholar] [CrossRef]
- Makarov, A.A.; Dzhemileva, L.U.; Salimova, A.R.; Makarova, E.K.; Ramazanov, I.R.; D’yakonov, V.A.; Dzhemilev, U.M. New Synthetic Derivatives of Natural 5Z,9Z-Dienoic Acids: Stereoselective Synthesis and Study of the Antitumor Activity. Bioorg. Chem. 2020, 104, 104303. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Dzhemileva, L.U.; Tuktarova, R.A.; Makarov, A.A.; Islamov, I.I.; Mulyukova, A.R.; Dzhemilev, U.M. Catalytic cyclometallation in steroid chemistry III: Synthesis of steroidal derivatives of 5Z,9Z-dienoic acid and investigation of its human topoisomerase I inhibitory activity. Steroids 2015, 102, 110–117. [Google Scholar] [CrossRef] [PubMed]
- D’yakonov, V.A.; Tuktarova, R.A.; Dzhemileva, L.U.; Ishmukhametova, S.R.; Yunusbaeva, M.M.; Dzhemilev, U.M. Catalytic cyclometallation in steroid chemistry V: Synthesis of hybrid molecules based on steroid oximes and (5Z,9Z)-tetradeca-5,9-dienedioic acid as potential anticancer agents. Steroids 2018, 138, 14–20. [Google Scholar] [CrossRef] [PubMed]
- D’yakonov, V.A.; Tuktarova, R.A.; Dzhemileva, L.U.; Ishmukhametova, S.R.; Yunusbaeva, M.M.; Dzhemilev, U.M. Catalytic cyclometallation in steroid chemistry VI: Targeted synthesis of hybrid molecules based on steroids and tetradeca-5Z,9Z-diene-1,14-dicarboxylic acid and study of their antitumor activity. Steroids 2018, 138, 6–13. [Google Scholar] [CrossRef] [PubMed]
- D’yakonov, V.A.; Dzhemileva, L.U.; Tuktarova, R.A.; Ishmukhametova, S.R.; Yunusbaeva, M.M.; Ramazanova, I.R.; Dzhemilev, U.M. Novel Hybrid Molecules on the Basis of Steroids and (5Z,9Z)-Tetradeca-5,9-dienoic Acid: Synthesis, Anti-Cancer Studies and Human Topoisomerase I Inhibitory Activity. Anticancer. Agents Med. Chem. 2017, 17, 1126–1135. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Kobayashi, K.; Furukawa, J.; Inagaki, J.; Sakairi, N.; Iwado, A.; Yasuda, T.; Koike, T.; Voelker, D.R.; Matsuura, E. Omega-carboxyl variants of 7-ketocholesteryl esters are ligands for beta(2)-glycoprotein I and mediate antibody-dependent uptake of oxidized LDL by macrophages. J. Lipid. Res. 2002, 43, 1486–1495. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Matsuura, E.; Liu, Q.; Furukawa, J.; Kaihara, K.; Inagaki, J.; Atsumi, T.; Sakairi, N.; Yasuda, T.; Voelker, D.R.; et al. A specific ligand for beta(2)-glycoprotein I mediates autoantibody-dependent uptake of oxidized low density lipoprotein by macrophages. J. Lipid. Res. 2001, 42, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T.J. Macrophages in Atherosclerosis Regression. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 20–33. [Google Scholar] [CrossRef]
- Michalik, L.; Auwerx, J.; Berger, J.P.; Chatterjee, V.K.; Glass, C.K.; Gonzalez, F.J.; Grimaldi, P.A.; Kadowaki, T.; Lazar, M.A.; O’Rahilly, S.; et al. International Union of Pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol Rev. 2006, 58, 726–741. [Google Scholar] [CrossRef]
- Dang, Z.; Jung, K.; Qian, K.; Lee, K.H.; Huang, L.; Chen, C.H. Synthesis of Lithocholic Acid Derivatives as Proteasome Regulators. ACS Med. Chem. Lett. 2012, 3, 925–930. [Google Scholar] [CrossRef]
- Sahara, H.; Hanashima, S.; Yamazaki, T.; Takahashi, S.; Sugawara, F.; Ohtani, S.; Ishikawa, M.; Mizushina, Y.; Ohta, K.; Shimozawa, K.; et al. Anti-tumor effect of chemically synthesized sulfolipids based on sea urchin’s natural sulfonoquinovosylmonoacylglycerols. Jpn. J. Cancer Res. 2002, 93, 85–92. [Google Scholar] [CrossRef]
- Mizushina, Y.; Kasai, N.; Miura, K.; Hanashima, S.; Takemura, M.; Yoshida, H.; Sugawara, F.; Sakaguchi, K. Structural relationship of lithocholic acid derivatives binding to the N-terminal 8-kDa domain of DNA polymerase beta. Biochemistry 2004, 43, 10669–10677. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.; Li, C.; Schmidt, E.J.; Boswell, J.S.; Walsh, J.P.; Allman, G.W.; Savage, P.B. Preparation and characterization of cholic acid-derived antimicrobial agents with controlled stabilities. Org. Lett. 2000, 2, 2837–2840. [Google Scholar] [CrossRef] [PubMed]
- Latha, R.M.; Lenin, M.; Rasool, M.; Varalakshmi, P. A novel derivative pentacyclic triterpene and ω 3 fatty acid [Lupeol-EPA] in relation to lysosomal enzymes glycoproteins and collagen in adjuvant induced arthritis in rats. Prostaglandins Leukot. Essent. Fat. Acids (PLEFA) 2001, 64, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Kweifio-Okai, G.; Field, B.; Rumble, B.A.; Macrides, T.A.; De Munk, F. Esterification improves antiarthritic effectiveness of Lupeol. Drug Dev. Res. 1995, 35, 137–141. [Google Scholar] [CrossRef]
- Kweifio-Okai, G.; De Munk, F.; Macrides, T.A.; Smith, P.; Rumble, B.A. Antiarthritic mechanisms of lupeol triterpenes. Drug Dev. Res. 1995, 36, 20–24. [Google Scholar] [CrossRef]
- Huang, L.; Chen, C.H. Molecular targets of anti-HIV-1 triterpenes. Curr. Drug Targets Infect. Disord. 2002, 2, 33–36. [Google Scholar] [CrossRef]
- Hashimoto, F.; Kashiwada, Y.; Cosentino, L.M.; Chen, C.H.; Garrett, P.E.; Lee, K.H. Anti-AIDS agents--XXVII. Synthesis and anti-HIV activity of betulinic acid and dihydrobetulinic acid derivatives. Bioorg. Med. Chem. 1997, 5, 2133–2143. [Google Scholar] [CrossRef]
- Kanamoto, T.; Kashiwada, Y.; Kanbara, K.; Gotoh, K.; Yoshimori, M.; Goto, T.; Sano, K.; Nakashima, H. Anti-human immunodeficiency virus activity of YK-FH312 (a betulinic acid derivative), a novel compound blocking viral maturation. Antimicrob. Agents Chemother. 2001, 45, 1225–1230. [Google Scholar] [CrossRef]
- Huang, L.; Yuan, X.; Aiken, C.; Chen, C.H. Bifunctional anti-human immunodeficiency virus type 1 small molecules with two novel mechanisms of action. Antimicrob. Agents Chemother. 2004, 48, 663–665. [Google Scholar] [CrossRef]
- Kashiwada, Y.; Nagao, T.; Hashimoto, A.; Ikeshiro, Y.; Okabe, H.; Cosentino, L.M.; Lee, K.H. Anti-AIDS agents 38. Anti-HIV activity of 3-O-acyl ursolic acid derivatives. J. Nat. Prod. 2000, 63, 1619–1622. [Google Scholar] [CrossRef]
- Ма, С.; Nacamura, N.; Hattori, М. Chemical modification of oleanene type triterpenes and their inhibitory activity against HIV-1 protease dimerisation. Chem. Pharm. Bull. 2000, 48, 1681–1688. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa-Goto, K.; Yamada, K.; Taniguchi, M.; Tokuda, H.; Lee, K.H. Cancer preventive agents 9. Betulinic acid derivatives as potent cancer chemopreventive agents. Bioorg. Med. Chem. Lett. 2009, 19, 3378–3381. [Google Scholar] [CrossRef] [PubMed]
- Pęcak, P.; Świtalska, M.; Chrobak, E.; Boryczka, G.; Bębenek, E. Betulin Acid Ester Derivatives Inhibit Cancer Cell Growth by Inducing Apoptosis through Caspase Cascade Activation: A Comprehensive In Vitro and In Silico Study. Int. J. Mol. Sci. 2022, 24, 196. [Google Scholar] [CrossRef] [PubMed]
- Kommera, H.; Kaluperović, G.N.K.; Kalbitz, J.; Paschke, R. Synthesis and Anticancer Activity of Novel Betulinic acid and Betulin Derivatives. Arch. Pharm. 2010, 8, 449–457. [Google Scholar] [CrossRef] [PubMed]
- D’yakonov, V.A.; Makarov, A.A.; Ibragimov, A.G.; Khalilov, L.M.; Dzhemilev, U.M. Novel Mg-Organic Reagents in Organic Synthesis. Cp2TiCl2 Catalyzed Intermolecular Cyclomagnesiation of Cyclic and Acyclic 1,2-Dienes Using Grignard Reagents. Tetrahedron 2008, 64, 10188–10194. [Google Scholar] [CrossRef]
- Dzhemileva, L.U.; D’yakonov, V.A.; Islamov, I.I.; Yunusbaeva, M.M.; Dzhemilev, U.M. New 1Z,5Z-diene macrodiolides: Catalytic synthesis, anticancer activity, induction of mitochondrial apoptosis, and effect on the cell cycle. Bioorg. Chem. 2020, 99, 103832. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Makarov, A.A.; Dzhemilev, U.M. Synthesis of gigantic macrocyclic polyketones through catalytic cyclometalation of cycloalkynes. Tetrahedron 2010, 66, 6885–6888. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Makarov, A.A.; Makarova, E.K.; Khalilov, L.M.; Dzhemilev, U.M. Synthesis and transformations of metallacycles 41. Cyclomagnesiation of O-containing 1,2-dienes with Grignard reagents in the presence of Cp2TiCl2. Russ. Chem. Bull. 2012, 61, 1943–1949. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Makarov, A.A.; Makarova EKh Dzhemilev, U.M. Novel organomagnesium reagents in synthesis. Catalytic cyclomagnesiation of allenes in the synthesis of N-, O-, and Si-substituted 1Z,5Z-dienes. Tetrahedron 2013, 69, 8516–8526. [Google Scholar] [CrossRef]
| Jurkat (IC50, µM) | K562 (IC50, µM) | U937 (IC50, µM) | Hek293 (IC50, µM) | Fibroblasts (IC50, µM) | |
|---|---|---|---|---|---|
| Compound 7 | 0.014 ± 0.002 | 0.007 ± 0.001 | 0.021 ± 0.003 | 0.127 ± 0.012 | 0.286 ± 0.022 |
| Compound 6 | 0.141 ± 0.012 | 0.159 ± 0.013 | 0.117 ± 0.012 | 0.253 ± 0.024 | 0.623 ± 0.058 |
| Oleanolic acid (5) | 0.196 ± 0.018 | 0.174 ± 0.014 | 0.131 ± 0.015 | 0.284 ± 0.027 | 0.698 ± 0.051 |
| Diol 4 | 0.383 ± 0.036 | 0.362 ± 0.031 | 0.299 ± 0.028 | 0.452 ± 0.044 | 0.874 ± 0.086 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuktarova, R.A.; Dzhemileva, L.U.; Dzhemilev, U.M. (5Z,9Z)-14-[(3,28-Dioxoolean-12-en-28-yl)oxy]tetradeca-5,9-dienoic Acid with Cytotoxic Activity. Molbank 2024, 2024, M1758. https://doi.org/10.3390/M1758
Tuktarova RA, Dzhemileva LU, Dzhemilev UM. (5Z,9Z)-14-[(3,28-Dioxoolean-12-en-28-yl)oxy]tetradeca-5,9-dienoic Acid with Cytotoxic Activity. Molbank. 2024; 2024(1):M1758. https://doi.org/10.3390/M1758
Chicago/Turabian StyleTuktarova, Regina A., Lilya U. Dzhemileva, and Usein M. Dzhemilev. 2024. "(5Z,9Z)-14-[(3,28-Dioxoolean-12-en-28-yl)oxy]tetradeca-5,9-dienoic Acid with Cytotoxic Activity" Molbank 2024, no. 1: M1758. https://doi.org/10.3390/M1758






