Coral Ecosystem Resilience, Conservation and Management on the Reefs of Jamaica in the Face of Anthropogenic Activities and Climate Change
Abstract
:1. Introduction

2. Methods
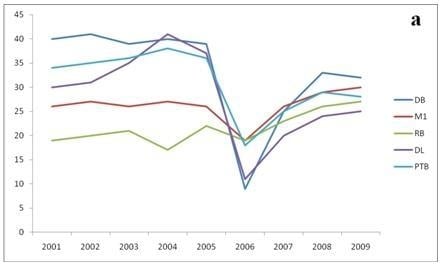
3. Results


| Year | Live coral (%) | Macroalgae (%) | Acropora species (%) |
|---|---|---|---|
| 2005 | 46 ± 8 | 8 ± 3 | 33 ± 5 |
| 2006 | 13 ± 5 | 6 ± 3 | 2 ± 2 |
| 2007 | 20 ± 9 | 6 ± 3 | 10 ± 4 |
| 2008 | 31 ± 7 | 5 ± 2 | 22 ± 7 |


4. Discussion-Reef Management and Conservation

- General Use
- Conservation Park
- Habitat Protection
- Marine National Park
- Another zone might be a Buffer Zone, next to a Marine National Park.
Acknowledgements
References
- Gardner, T.A.; Côté, I.M.; Gill, J.A.; Grant, A.; Watkinson, A.R. Long-term region-wide declines in Caribbean corals. Science 2003, 301, 958–960. [Google Scholar] [CrossRef]
- Bellwood, D.R.; Hughes, T.P.; Folke, C.; Nyström, M. Confronting the coral reef crisis. Nature 2004, 429, 827–833. [Google Scholar] [CrossRef]
- Connell, J.H. Diversity in tropical rain forests and coral reefs. Science 1978, 199, 1302–1310. [Google Scholar]
- Rogers, C.S. Hurricanes and coral reefs: the intermediate disturbance hypothesis revisited. Coral Reef. 1993, 12, 127–137. [Google Scholar] [CrossRef]
- Stoll, P.; Prati, D. Intraspecific aggregation alters competitive interactions in experimental plant communities. Ecology 2001, 82, 319–327. [Google Scholar] [CrossRef]
- Hartley, S.; Shorrocks, B. A general framework for the aggregation model of coexistence. J. Anim. Ecol. 2002, 71, 651–662. [Google Scholar] [CrossRef]
- Karlson, R.H. Dynamics of Coral Communities; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1999. [Google Scholar]
- Karlson, R.H.; Cornell, H.V.; Hughes, T.P. Aggregation influences coral species richness at multiple spatial scales. Ecology 2007, 88, 170–177. [Google Scholar] [CrossRef]
- Lirman, D. Competition between macroalgae and corals: Effects of herbivore exclusion and increased algal biomass on coral survivorship and growth. Coral Reef. 2001, 19, 392–399. [Google Scholar] [CrossRef]
- Box, S.J.; Mumby, P.J. Effect of macroalgal competition on growth and survival of juvenile Caribbean corals. Mar. Ecol. Prog. Ser. 2007, 342, 139–149. [Google Scholar] [CrossRef]
- Idjadi, J.A.; Karlson, R.H. Spatial arrangement of competitors influences coexistence of reef-building corals. Ecology 2007, 88, 2449–2454. [Google Scholar] [CrossRef]
- Crabbe, M.J.C. Topography and spatial arrangement of reef-building corals on the fringing reefs of North Jamaica may influence their response to disturbance from bleaching. Mar. Environ. Res. 2010, 69, 158–162. [Google Scholar] [CrossRef]
- Hughes, T.P.; Tanner, J.E. Recruitment failure, life histories and long-term decline of Caribbean corals. Ecology 2000, 81, 2250–2263. [Google Scholar] [CrossRef]
- Coles, S.L.; Brown, E.K. Twenty-five years of change in coral coverage on a hurricane impacted reef in Hawai'i: the importance of recruitment. Coral Reef. 2007, 26, 705–717. [Google Scholar] [CrossRef]
- Mumby, P.J.; Hastings, A.; Edwards, H.J. Thresholds and the resilience of Caribbean coral reefs. Nature 2007, 450, 98–101. [Google Scholar] [CrossRef]
- Stat, M.; Morris, E.; Gates, R.D. Functional diversity in coral-dinoflagellate symbiosis. Proc. Natl. Acad. Sci. USA 2008, 105, 9256–9261. [Google Scholar] [CrossRef]
- Mora, C. A clear human footprint in the coral reefs of the Caribbean. Proc. R. Soc. Lond. B Biol. Sci. 2008, 275, 767–773. [Google Scholar] [CrossRef]
- Soong, K. Colony size as species character in massive reef corals. Coral Reef. 1993, 12, 77–83. [Google Scholar] [CrossRef]
- Meesters, E.H.I.; Hilterman, M.; Kardinaal, E.; Keetman, M.; de Vries, M.; Bak, R.P.M. Colony size-frequency distributions of scleractinian coral populations: spatial and interspecific variation. Mar. Ecol. Prog. Ser. 2001, 209, 43–54. [Google Scholar] [CrossRef]
- Smith, L.D.; Devlin, M.; Haynes, D.; Gilmour, J.P. A demographic approach to monitoring the health of coral reefs. Mar. Pollut. Bull. 2005, 51, 399–407. [Google Scholar] [CrossRef]
- Woodley, J.D.; Chornesky, E.A.; Clifford, P.A.; Jackson, J.B.C.; Kaufman, L.S.; Knowlton, N.; Lang, J.C.; Pearson, M.P.; Porter, J.W.; Rooney, M.C.; Rylaarsdam, K.W.; Tunnicliffe, V.J.; Wahle, C.M.; Wulff, J.L.; Curtis, A.S.G.; Dallmeyer, M.D.; Jupp, B.P.; Koehl, M.A.R.; Neigel, J.; Sides, E.M. Hurricane Allen’s impact on Jamaican coral reefs. Science 1981, 214, 749–755. [Google Scholar]
- Crabbe, M.J.C.; Mendes, J.M.; Warner, G.F. Lack of recruitment of non-branching corals in Discovery Bay is linked to severe storms. Bull. Mar. Sci. 2002, 70, 939–945. [Google Scholar]
- Jackson, J.B.C. Reefs since Columbus. Proc. 8th. Int. Coral Reef Symp. 1997, 1, 97–106. [Google Scholar]
- Hawkins, J.P.; Roberts, C.M. Effects of artisanal fishing on Caribbean coral reefs. Conserv. Biol. 2004, 18, 215–226. [Google Scholar] [CrossRef]
- Hughes, T.P. Catastrophes, phase shifts and large-scale degradation of a Caribbean coral reef. Science 1994, 265, 1547–1551. [Google Scholar]
- Aronson, R.B.; Precht, W.F. Evolutionary paleoecology of Caribbean coral reefs. In Evolutionary Paleoecology: The Ecological Context of Macroevolutionary Change; Allmon, W.D., Bottjer, D.J., Eds.; Columbia University Press: New York, NY, USA, 2001; pp. 171–233. [Google Scholar]
- Greenaway, A.M.; Gordon-Smith, D.-A. The effects of rainfall on the distribution of inorganic nitrogen and phosphorus in Discovery Bay, Jamaica. Limnol. Oceanogr. 2006, 51, 2206–2220. [Google Scholar] [CrossRef]
- Bruno, J.F.; Sweatman, H.; Precht, W.F.; Selig, E.R.; Schutte, V.G.W. Assessing evidence of phase shifts from coral to macroalgal dominance on coral reefs. Ecology 2009, 90, 1478–1484. [Google Scholar] [CrossRef]
- Liu, P.-J.; Shao, K.-T.; Jan, R.-Q.; Fan, T.-Y.; Wong, S.-L.; Hwang, J.-S.; Chen, J.-P.; Chen, C.-C.; Lin, H.-J. A trophic model of fringing coral reefs in Nanwan Bay, southern Taiwan suggest overfishing. Mar. Environ. Res. 2009, 68, 106–117. [Google Scholar] [CrossRef]
- Jones, L.; Alcolado, P.M.; Cala, Y.; Cobián, D.; Coelho, V.; Hernández, A.; Jones, R.; Mallela, J.; Manfrino, C. The effects of coral bleaching in the northern Caribbean and western Atlantic. In Status of Caribbean Coral Reefs after Bleaching and Hurricanes in 2005; Wilkinson, C., Souter, D., Eds.; Global Coral Reef Monitoring Network, and Reef and Rainforest Research Centre: Townsville, Australia, 2008; pp. 73–83. [Google Scholar]
- Wilkinson, C.; Souter, D. Status of Caribbean Coral Reefs after Bleaching and Hurricanes in 2005; Global Coral Reef Monitoring Network, and Reef and Rainforest Research Centre: Townsville, Australia, 2008. [Google Scholar]
- Quinn, N.J.; Kojis, B.L. The recent collapse of a rapid phase-shift reversal on a Jamaican north coast reef after the 2005 bleaching event. Int. J. Trop. Biol. 2008, 56, 149–159. [Google Scholar]
- Crabbe, M.J.C. Scleractinian coral population size structures and growth rates indicate coral resilience on the fringing reefs of North Jamaica. Mar. Environ. Res. 2009, 67, 189–198. [Google Scholar] [CrossRef]
- Idjadi, J.A.; Lee, S.C.; Bruno, J.F.; Precht, W.F.; Allen-Requa, L.; Edmunds, P.J. Rapid phase-shift reversal on a Jamaican coral reef. Coral Reef. 2006, 25, 209–211. [Google Scholar] [CrossRef]
- Meesters, E.H.I.; Hilterman, M.; Kardinaal, E.; Keetman, M.; de Vries, M.; Bak, R.P.M. Colony size-frequency distributions of scleractinian coral populations: spatial and interspecific variation. Mar. Ecol. Prog. Ser. 2001, 209, 43–54. [Google Scholar] [CrossRef]
- Loya, Y. Skeletal regeneration in a Red Sea scleractinian coral population. Nature 1976, 261, 490–491. [Google Scholar] [CrossRef]
- Abrego, D.; Ulstrup, K.E.; Willis, B.L.; van Oppen, M.J.H. Species-specific interactions between algal endosymbionts and coral hosts define their bleaching response to heat and light stress. Proc. R. Soc. Lond. B Biol. Sci. 2008, 275, 2273–2282. [Google Scholar] [CrossRef]
- Donner, S.D.; Knutson, T.R.; Oppenheimer, M. Model-based assessment of the role of human-induced climate change in the 2005 Caribbean coral bleaching event. Proc. Natl. Acad. Sci. USA 2007, 104, 5483–5488. [Google Scholar] [CrossRef]
- Morgan, J.; Heron, S.; Eakin, M. The 2005 bleaching event: coral-list log. In Status of Caribbean Coral Reefs after Bleaching and Hurricanes in 2005; Wilkinson, C., Souter, D., Eds.; Global Coral Reef Monitoring Network, and Reef and Rainforest Research Centre: Townsville, Australia, 2008; pp. 37–44. [Google Scholar]
- Mallela, J.; Crabbe, M.J.C. Hurricanes and coral bleaching linked to changes in coral recruitment in Tobago. Mar. Environ. Res. 2009, 68, 158–162. [Google Scholar] [CrossRef]
- Nyström, M.; Graham, N.A.J.; Lokrantz, J.; Norström, A.V. Capturing the cornerstones of coral reef resilience: linking theory to practice. Coral Reef. 2008, 27, 795–809. [Google Scholar] [CrossRef]
- Halford, A.R.; Caley, M.J. Towards an understanding of resilience in isolated coral reefs. Glob. Change Biol. 2009, 15, 3031–3045. [Google Scholar] [CrossRef]
- Alvarez-Filip, L.; Dulvy, N.K.; Gill, J.A.; Côté, I.M.; Watkinson, A.R. Flattening of Caribbean coral reefs: region-wide declines in architectural complexity. Proc. R. Soc. Lond. B Biol. Sci. 2009, 276, 3019–3025. [Google Scholar] [CrossRef]
- Adjeroud, M.; Michonneau, F.; Edmunds, P.J.; Chancerelle, Y.; de Loma, T.L.; Penin, L.; Thibaut, L.; Vidal-Dupiol, J.; Salvat, B.; Galzin, R. Recurrent disturbances, recovery trajectories, and resilience of coral assemblages on a South Central Pacific reef. Coral Reef. 2009, 28, 775–780. [Google Scholar] [CrossRef]
- Cooper, T.F.; Gilmour, J.P.; Fabricius, K.E. Bioindicators of changes in water quality on coral reefs: review and recommendations for monitoring programmes. Coral Reef. 2009, 28, 589–606. [Google Scholar] [CrossRef]
- Chaves, L.D.T.; Monteiro-Neto, C. Comparative analysis of rocky reef fish community structure in coastal islands of south-eastern Brazil. J. Mar. Biol. Assoc. UK 2009, 89, 609–619. [Google Scholar] [CrossRef]
- Howard, K.G.; Schumacher, B.D.; Parrish, J.D. Community structure and habitat associations of parrotfishes on Oahu, Hawaii. Environ. Biol. Fish. 2009, 85, 175–186. [Google Scholar] [CrossRef]
- Emslie, M.J.; Cheal, A.J.; Sweatman, H.; Delean, S. Recovery from disturbance of coral and reef fish communities on the Great Barrier Reef, Australia. Mar. Ecol. Prog. Ser. 2008, 371, 177–190. [Google Scholar] [CrossRef]
- Chapman, M.R.; Kramer, D.L. Gradients in coral reef fish density and size across the Barbados Marine reserve boundary: effects of reserve protection and habitat characteristics. Mar. Ecol. Prog. Ser. 1999, 181, 81–96. [Google Scholar] [CrossRef]
- Wilson, S.K.; Graham, N.A.J.; Polunin, N.V.C. Appraisal of visual assessments of habitat complexity and benthic composition on coral reefs. Mar. Biol. 2007, 151, 1069–1076. [Google Scholar] [CrossRef]
- Rooker, J.R.; Dokken, Q.R.; Pattengill, C.V.; Holt, G.J. Fish assemblages on artificial and natural reefs in the Flower Garden Banks National Marine Sanctuary, USA. Coral Reef. 1997, 16, 83–92. [Google Scholar] [CrossRef]
- Macia, S.; Robinson, M.P.; Nalevanko, A. Experimental dispersal of recovering Diadema antillarum increases grazing intensity and reduces macroalgal abundance on a coral reef. Mar. Ecol. Prog. Ser. 2007, 348, 173–182. [Google Scholar] [CrossRef]
- Dunn, D.C.; Halpin, P.N. Rugosity-based regional modelling of hard bottom habitat. Mar. Ecol. Prog. Ser. 2009, 377, 1–11. [Google Scholar] [CrossRef]
- Anthony, K.R.N.; Kline, D.I.; Diaz-Pulido, G.; Dove, S.; Hoegh-Guldberg, O. Ocean acidification causes bleaching and productivity loss in coral reef builders. Proc. Natl. Acad. Sci. USA 2008, 105, 17442–17446. [Google Scholar]
- Kinzig, A.P.; Pacala, S.W. Successional biodiversity and ecosystem functioning. In The Functional Consequences of Biodiversity; Kinzig, A.P., Pacala, S.W., Tilman, D., Eds.; Princeton University Press: Princeton, NJ, USA, 2001; pp. 175–212. [Google Scholar]
- Brockhurst, M.A.; Colegrave, N.; Hodgson, D.J.; Buckling, A. Niche occupation limits adaptive radiation in experimental microcosms. PLoS One 2007, 2, e193. [Google Scholar] [CrossRef]
- Thompson, D.M.; van Woesik, R. Corals escape bleaching in regions that recently and historically experienced frequent thermal stress. Proc. R. Soc. Lond. B Biol. Sci. 2009, 276, 2931–2940. [Google Scholar] [CrossRef]
- Wescott, G. Partnerships for capacity building: community, governments and universities working together. Ocean Coast. Manage. 2002, 45, 549–571. [Google Scholar] [CrossRef]
- Balgos, M. Integrated coastal management and marine protected areas in the Philippines: concurrent developments. Ocean Coast. Manage. 2005, 48, 972–995. [Google Scholar] [CrossRef]
- Chircop, A. Introduction to capacity building. Ocean Coast. Manage. 1998, 38, 67–68. [Google Scholar] [CrossRef]
- Crabbe, M.J.C.; Martinez, E.; Garcia, C.; Chub, J.; Castro, L.; Guy, J. Is capacity building important in policy development for sustainability? A case study using action plans for sustainable Marine Protected Areas in Belize. Soc. Nat. Resource. 2010, 23, 181–190. [Google Scholar]
- Mutandwa, E.; Gadzirayi, C. Impact of community-based approaches to wildlife management: a case study of the CAMPFIRE programme in Zimbabwe. Int. J. Sustain. Dev. World Ecol. 2007, 14, 336–344. [Google Scholar] [CrossRef]
- Tai, H.S. Development through conservation: an institutional analysis of indigenous community-based conservation in Taiwan. World Develop. 2007, 35, 1186–1203. [Google Scholar] [CrossRef]
- Allen, K. Community-based disaster preparedness and climate adaptation: local capacity-building in the Philippines. Disasteres 2006, 30, 81–101. [Google Scholar] [CrossRef]
- Stojanovic, T.; Barker, N. Improving governance through local coastal partnerships in the UK. Geog. J. 2008, 174, 344–360. [Google Scholar] [CrossRef]
- Williams, I.; Polunin, N. Differences between protected and unprotected reefs of the Western Caribbean in attributes preferred by dive tourists. Environ. Cons. 2000, 27, 382–391. [Google Scholar]
- Cho, L. Marine protected areas: a tool for integrated coastal management in Belize. Ocean Coast. Manage. 2005, 48, 932–947. [Google Scholar] [CrossRef]
- Mumby, P.J; Harborne, A.R. Marine reserves enhance the recovery of corals on Caribbean reefs. PLoS One 2010, 5, e8657. [Google Scholar] [CrossRef]
- Hills, J.; Alcock, D.; Higham, T.; Kirkman, H.; LeTissier, M.; Pagdilao, C.; Samonte, P.; Smith, T. Capacity building for Intergrated Coastal management in Asia-Pacific: the case for case studies. Coast. Manage. 2006, 34, 323–337. [Google Scholar] [CrossRef]
- Christie, P.; White, A. Best practices for improved governance of coral reef marine protected areas. Coral Reef. 2007, 26, 1047–1056. [Google Scholar] [CrossRef]
- Johnson, T.; van Densen, W. Benefits and organisation of cooperative research for fisheries management. ICES J. Mar. Sci. 2007, 64, 834–840. [Google Scholar] [CrossRef]
- Poulsen, S. Examples of capacity building cooperation. Waste Manage. Res. 2007, 25, 283–287. [Google Scholar] [CrossRef]
- Mumby, P.; Steneck, R. Coral reef management and conservation in light of rapidly evolving ecological paradigms. Trends Ecol. Evol. 2008, 23, 555–563. [Google Scholar] [CrossRef]
- Coffin, B. Building ethical capacity for collaborative research. Nonprofit Volunt. Sect. Q. 2005, 34, 531–539. [Google Scholar] [CrossRef]
- Mow, J.; Taylor, E.; Howard, M.; Baine, M.; Connolly, E.; Chiquillo, M. Collaborative planning and management of the San Andres Archipelago’s coastal and marine resources: a short communication on the evolution of the Seaflower Marine Protected Area. Ocean Coast. Manage. 2007, 50, 209–222. [Google Scholar] [CrossRef]
- Norris-Raynbird, C. Mediation-fostered equality?—An examination of environmental negotiations from a power/process perspective. Proc. Gulf Carib. Fish. Inst. 2004, 55, 155–177. [Google Scholar]
- TEEB—The Economics of Ecosystems and Biodiversity for national and international policymakers—Summary: Responding to the value of nature. 2009, p. 47. Available online: http://www.unep.org/Documents.Multilingual/Default.asp?DocumentID=602&ArticleID=6371&l=en&t=long (accessed on 1 March 2010).
- Burke, L.; Selig, E.; Spalding, M. Reefs at Risk in Southeast Asia; World Resources Institute (WRI): Washington, DC, USA. Available online: http://www.wri.org (accessed on 8 March 2010).
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Crabbe, M.J.C. Coral Ecosystem Resilience, Conservation and Management on the Reefs of Jamaica in the Face of Anthropogenic Activities and Climate Change. Diversity 2010, 2, 881-896. https://doi.org/10.3390/d2060881
Crabbe MJC. Coral Ecosystem Resilience, Conservation and Management on the Reefs of Jamaica in the Face of Anthropogenic Activities and Climate Change. Diversity. 2010; 2(6):881-896. https://doi.org/10.3390/d2060881
Chicago/Turabian StyleCrabbe, M. James C. 2010. "Coral Ecosystem Resilience, Conservation and Management on the Reefs of Jamaica in the Face of Anthropogenic Activities and Climate Change" Diversity 2, no. 6: 881-896. https://doi.org/10.3390/d2060881