The Effects of Salinity and pH on Fertilization, Early Development, and Hatching in the Crown-of-Thorns Seastar
Abstract
:1. Introduction
2. Methods
2.1. Adult Collection and Maintenance
2.2. Effects of Salinity on Fertilization, Development, and Hatching
2.3. Effects of Salinity and pH on Development to Hatching
2.4. Statistical Analysis
3. Results
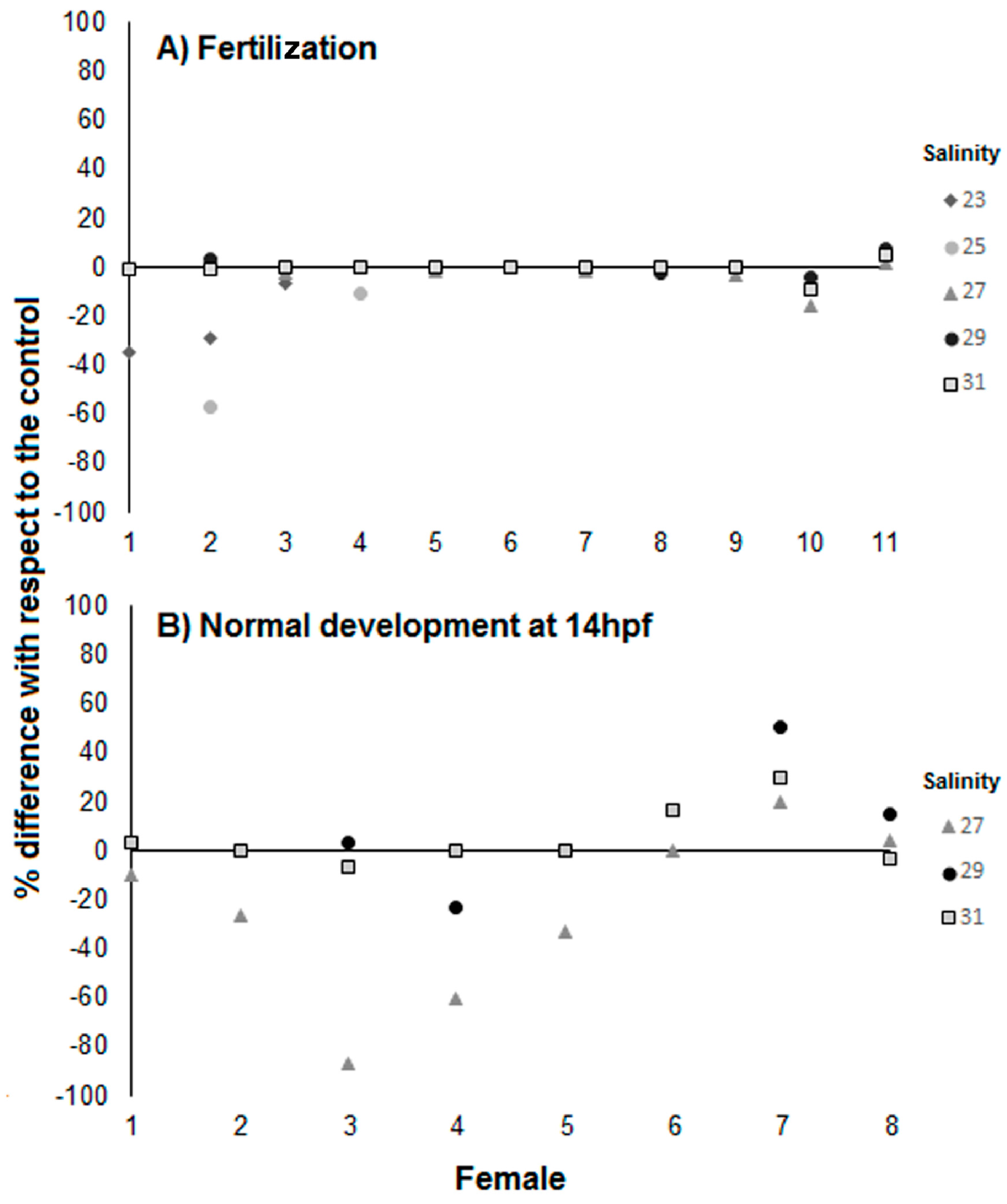
3.1. Effects of Salinity on Fertilization, Early Development, and Hatching Time of Crown-of-Thorns Seastars
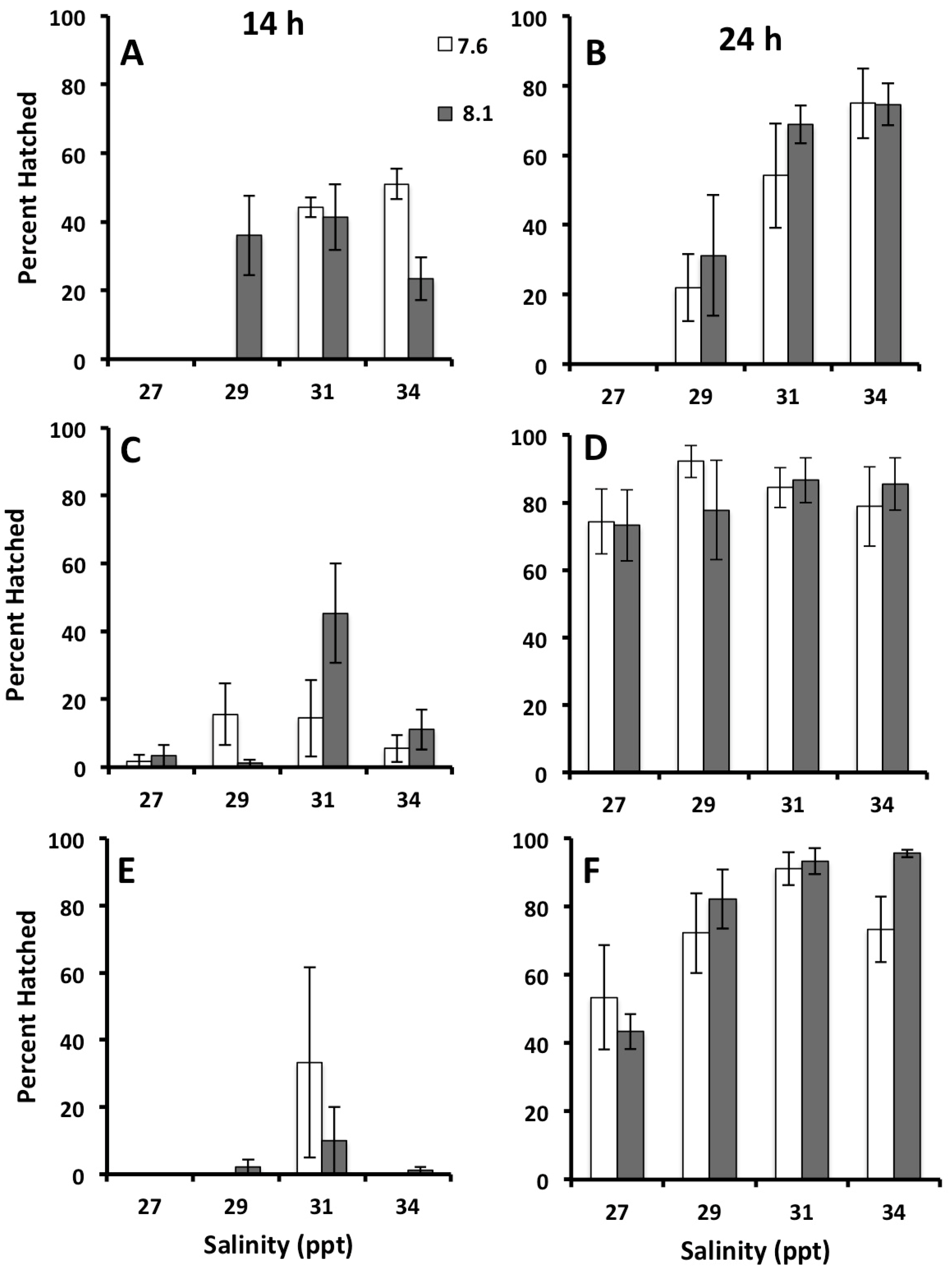
3.2. Effects of Salinity and pH on Early Development of Crown of Thorns Seastars
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Anthony, K.R.N. Coral reefs under climate change and ocean acidification: Challenges and opportunities for management and policy. Ann. Rev. Environ. Resour. 2016, 41, 59–81. [Google Scholar] [CrossRef]
- Lough, J.M. Coral reefs: Turning back time. Nature 2016, 531, 314–315. [Google Scholar] [CrossRef] [PubMed]
- Normile, D. El Niño’s warmth devastating reefs worldwide. Science 2016, 352, 15–16. [Google Scholar] [CrossRef] [PubMed]
- Bruno, J.F.; Selig, E.R. Regional Decline of Coral Cover in the Indo-Pacific: Timing, Extent, and Subregional Comparisons. PLoS ONE 2007, 2, e711. [Google Scholar] [CrossRef] [PubMed]
- De’ath, G.; Fabricius, K.E.; Sweatman, H.; Puotinen, M. The 27—Year decline of coral cover on the Great Barrier Reef and its causes. Proc. Nat. Acad. Sci. USA 2012, 109, 17995–17999. [Google Scholar] [CrossRef] [PubMed]
- Baird, A.H.; Pratchett, M.S.; Hoey, A.S.; Herdiana, Y.; Campbell, S.J. Acanthaster planci is a major cause of coral mortality in Indonesia. Coral Reefs 2013, 32, 803–812. [Google Scholar] [CrossRef]
- Plass-Johnson, J.G.; Schwieder, H.; Heiden, J.; Weiand, L.; Wild, C.; Jompa, J.; Ferse, S.C.A.; Teichberg, M. A recent outbreak of crown-of-thorns starfish (Acanthaster planci) in the Spermonde Archipelago, Indonesia. Reg. Environ. Chang. 2015, 15, 1157–1162. [Google Scholar] [CrossRef]
- Pratchett, M.S.; Caballes, C.F.; Rivera-Posada, J.A.; Sweatman, H.P.A. Limits to Understanding and Managing Outbreaks of Crown-of-Thorns Starfish (Acanthaster spp.). Oceanogr. Mar. Biol. Annu. Rev. 2014, 52, 133–200. [Google Scholar]
- Osborne, K.; Dolman, A.M.; Burgess, S.C.; Kerryn, J.A. Disturbance and dynamics of coral cover on the Great Barrier Reef (1995–2009). PLoS ONE 2011, 6, e17516. [Google Scholar] [CrossRef] [PubMed]
- Lourey, M.J.; Ryan, D.A.J.; Miller, I.R. Rates of decline and recovery of coral cover on reefs impacted by, recovering from and unaffected by crown-of-thorns starfish Acanthaster planci: A regional perspective of the Great Barrier Reef. Mar. Ecol. Prog. Ser. 2000, 196, 179–186. [Google Scholar] [CrossRef]
- Halford, A.; Cheal, A.J.; Ryan, D.; Williams, D.M. Resilience to large-scale disturbance in coral and fish assemblages on the Great Barrier Reef. Ecology 2004, 85, 1892–1905. [Google Scholar] [CrossRef]
- Fabricius, K.E.; Okaji, K.; De’ath, G. Three lines of evidence to link outbreaks of the crown-of-thorns seastar Acanthaster planci to the release of larval food limitation. Coral Reefs 2010, 29, 593–605. [Google Scholar] [CrossRef]
- Kayal, M.; Vercelloni, J.; Lison de Loma, T.; Bosserelle, P.; Chancerelle, Y.; Geoffroy, S.; Stievenart, C.; Michonneau, F.; Penin, L.; Planes, S.; et al. Predator Crown-of-Thorns Starfish (Acanthaster planci) Outbreak, Mass Mortality of Corals, and Cascading Effects on Reef Fish and Benthic Communities. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Uthicke, S.; Schaffelke, B.; Byrne, M. A boom-bust phylum? Ecological and evolutionary consequences of density variations in echinoderms. Ecol. Monogr. 2009, 79, 3–24. [Google Scholar] [CrossRef]
- Wolfe, K.; Graba-Landry, A.; Dworjanyn, S.A.; Byrne, M. Larval phenotypic plasticity in the boom-and-bust crown-of-thorns seastar, Acanthaster planci. Mar. Ecol. Prog. Ser. 2015, 539, 179–189. [Google Scholar] [CrossRef]
- Wolfe, K.; Graba-Landry, A.; Dworjanyn, S.A.; Byrne, M. Larval starvation to satiation: influence of nutrient regime on the success of Acanthaster planci. PLoS ONE 2015, 10, e0122010. [Google Scholar] [CrossRef] [PubMed]
- Babcock, R.C.; Milton, D.A.; Pratchett, M.S. Relationships between size and reproductive output in the crown-of-thorns starfish. Mar. Biol. 2016, 163, 234. [Google Scholar] [CrossRef]
- Wooldridge, S.A.; Brodie, J.E. Environmental triggers for primary outbreaks of crown-of-thorns star fish on the Great Barrier Reef, Australia. Mar. Pollut. Bull. 2015, 101, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, K.; Graba-Landry, A.; Dworjanyn, S.A.; Byrne, M. Superstars: Assessing nutrient thresholds for enhance larval success of Acanthaster planci, a review of the evidence. Mar. Pollut. Bull. 2017. [Google Scholar] [CrossRef] [PubMed]
- Brodie, J.; Fabricius, K.; De’ath, G.; Okaji, K. Are increased nutrient inputs responsible for more outbreaks of crown-of-thorns starfish? An appraisal of the evidence. Mar. Pollut. Bull. 2005, 51, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Wolanski, E.; Jones, M. Physical properties of Great Barrier Reef lagoon waters near Townsville. I. Effects of Burdekin River floods. Mar. Freshw. Res. 1981, 32, 305–319. [Google Scholar] [CrossRef]
- King, B.A.; McAllister, F.A.; Wolanski, E.J.; Done, T.J.; Spagnol, S.B. River plume dynamics in the central Great Barrier Reef. In Oceanographic Processes of Coral Reefs: Physical and Biological Links in the Great Barrier Reef; Wolanski, E.J., Ed.; CRC Press: Boca Raton, FL, USA, 2001; Chapter 10; pp. 145–160, 356. [Google Scholar]
- Brodie, J.E.; Kroon, F.J.; Schaffelke, B.; Wolanski, E.C.; Lewis, S.E.; Devlin, M.J.; Bohnet, I.C.; Bainbridge, Z.T.; Waterhouse, J.; Davis, A.M. Terrestrial pollutant runoff to the Great Barrier Reer: An update of issues, priorities and management responses. Mar. Pollut. Bull. 2012, 65, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Lough, J.M.; Lewis, S.E.; Cantin, N.E. Freshwater impacts in the central Great Barrier Reef: 1648–2011. Coral Reefs 2015, 34, 739–751. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Stickle, W.B.; Diehl, W.J. Effects of salinity on echinoderms. In Echinoderm Studies; Jangoux, M., Lawrence, J.M., Eds.; Balkema: Rotterdam, The Netherlands, 1987; Volume 2, pp. 235–285. [Google Scholar]
- Lucas, J. Reproductive and larval biology of Acanthaster planci (L.) in Great Barrier Reef waters. Micronesica 1973, 9, 197–203. [Google Scholar]
- Russell, M.P. Echinoderm responses to variation in salinity. Adv. Mar. Biol. 2013, 66, 171–212. [Google Scholar] [PubMed]
- Allen, J.D.; Pechenik, J.A. Understanding the effects of low salinity on fertilization success and early development in the sand dollar Echinarachnius parma. Biol. Bull. 2010, 218, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.F.; Blackburn, H.N.; Allen, J.D. A novel report of hatching plasticity in the phylum Echinodermata. Am. Nat. 2013, 181, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.C.; Sewell, M.A.; Matson, P.G.; Rivest, E.B.; Kapsenberg, L.; Hofmann, G.E. Growth attenuation with developmental schedule progression in embryos and early larvae of Sterechinus neumayeri raised under elevated CO2. PLoS ONE 2013, 8, e52448. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.D.; Armstrong, A.F.; Ziegler, S.L. Environmental induction of polyembryony in echinoid echinoderms. Biol. Bull. 2015, 229, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Dickson, A.G.; Sabine, C.L.; Christian, J.R. Guide to Best Practices for Ocean O2 Measurements; PICES Special Publication: Sidney, Canada, 2007; Volume 3, p. 119. [Google Scholar]
- Liu, X.; Patsavas, M.C.; Byrne, R.H. Purification and characterization of meta-cresol purple for spectrophotometric seawater pH measurements. Environ. Sci. Technol. 2011, 45, 4862–4868. [Google Scholar] [CrossRef] [PubMed]
- Pierrot, D.; Lewis, E.; Wallace, D.W.R. MS Excel Program Developed for CO2 System Calculations. ORNL/CDIAC-105a; Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US Department of Energy: Oak Ridge, TE, USA, 2006.
- Mehrbach, C.; Culberson, C.H.; Hawley, J.E.; Pytkowicz, R.M. Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol. Oceanogr. 1973, 18, 897–907. [Google Scholar] [CrossRef]
- Dickson, A.G.; Millero, F.J. A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep Sea Res. 1987, 34, 1733–1743. [Google Scholar] [CrossRef]
- Kramer, A.A.; Zimmerman, J.E. Assessing the calibration of mortality benchmarks in critical care: The Hosmer-Lemeshow test revisited. Crit. Care Med. 2007, 35, 2052–2056. [Google Scholar] [CrossRef] [PubMed]
- Dunn, O.J. Multiple Comparisons among Means. J. Am. Stat. Assoc. 1961, 56, 52–64. [Google Scholar] [CrossRef]
- Dunn, O.J. Multiple Comparisons Using Rank Sums. Technometrics 1964, 6, 241–252. [Google Scholar] [CrossRef]
- Quinn, G.P.; Keough, M.J. Experimental Design and Data Analysis for Biologists; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Kashenko, S.D. Responses of embryos and larvae of the starfish Asterias amurensis to changes in temperature and salinity. Russ. J. Mar. Biol. 2005, 31, 294–302. [Google Scholar] [CrossRef]
- Kashenko, S.D. The combined effect of temperature and salinity on development of the sea star Asterina pectinifera. Russ. J. Mar. Biol. 2006, 32, 37–44. [Google Scholar] [CrossRef]
- Caballes, C.F.; Pratchett, M.S.; Raymundo, M.L.; Rivera-Posada, J.A. Environmental tipping points for sperm motility, fertilization, and embryonic development in the Crown-of-Thorns starfish, Acanthaster cf. solaris. Diversity 2017, 9, 10. [Google Scholar]
- Roller, R.A.; Stickle, W.B. Effects of temperature and salinity acclimation of adults on larval survival, physiology, and early development of Lytechinus variegatus. Mar. Biol. 1993, 116, 583–591. [Google Scholar] [CrossRef]
- Carballeira, C.; Martin-Diaz, L.; DelValls, T.A. Influence of salinity on fertilization and larval development toxicity tests with two species of sea urchin. Mar. Environ. Res. 2011, 72, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Delorme, N.J.; Sewell, M.A. Temperature and salinity: Two climate change stressors affecting early development of the New Zealand sea urchin Evechinus chloroticus. Mar. Biol. 2014, 161, 1999. [Google Scholar] [CrossRef]
- Foo, S.A.; Byrne, M. Acclimatization and adaptive capacity of marine species in a changing ocean. Adv. Mar. Biol. 2016, 74, 69–116. [Google Scholar] [PubMed]
- Roller, R.A.; Stickle, W.B. Effects of salinity on larval tolerance and early developmental rates of 4 species of echinoderms. Can. J. Zool. 1985, 63, 1531–1538. [Google Scholar] [CrossRef]
- Metaxas, A. The effect of salinity on larval survival and development in the sea urchin Echinometra lucunter. Invertebr. Reprod. Dev. 1998, 34, 323–330. [Google Scholar] [CrossRef]
- Schiopu, D.; George, S.B. Diet and salinity effects on larval growth and development of the sand dollar Mellita isometra. Invertebr. Reprod. Dev. 2004, 45, 69–82. [Google Scholar] [CrossRef]
- Cowart, D.A.; Ulrich, P.N.; Miller, D.C.; Marsh, A.G. Salinity sensitivity of early embryos of the Antarctic sea urchin Sterechinus neumayeri. Pol. Biol. 2009, 32, 435–441. [Google Scholar] [CrossRef]
- Watts, S.A.; Scheibling, R.E.; Marsh, A.G.; McClintock, J.B. Effect of tempertature and salinity on larval development of sibling species of Echinaster (Echinodermata, Asteroidea) and their hybrids. Biol. Bull. 1982, 163, 348–354. [Google Scholar] [CrossRef]
- Morgan, S.G. Life and death in the plankton: Larval mortality and adaptation. In Ecology of Marine Invertebrate Larvae; McEdward, L.R., Ed.; CRC Press: Boca Raton, FL, USA, 1995; pp. 279–321. [Google Scholar]
- Lamare, M.; Barker, M.F. In situ estimates of larval development and mortality in the New Zealan sea urchin Evechinus chloroticus (Echinodermata: Echinoidea). Mar. Ecol. Prog. Ser. 1999, 180, 197–211. [Google Scholar] [CrossRef]
- Vaughn, D.; Allen, J.D. The peril of the plankton. Int. Comp. Biol. 2010, 50, 552–570. [Google Scholar] [CrossRef] [PubMed]
- Uthicke, S.; Pecorino, D.; Albright, R.; Negri, A.P.; Cantin, N.; Liddy, M.; Dworjanyn, S.; Kamya, P.; Byrne, M.; Lamare, M. Impacts of ocean acidification on early life-history stages and settlement of the coral-eating sea star Acanthaster planci. PLoS ONE 2013, 8, e8293. [Google Scholar] [CrossRef] [PubMed]
- Byrne, M. Global change ecotoxicology: Identification of early life history bottlenecks in marine invertebrates, variable species responses and variable experimental approaches. Mar. Environ. Res. 2012, 76, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Kamya, P.Z.; Dworjanyn, S.A.; Hardy, N.; Mos, B.; Uthicke, S.; Byrne, M. Larvae of the coral eating crown-of-thorns starfish, Acanthaster planci in a warmer high CO2 ocean. Glob. Chang. Biol. 2014, 20, 3365–3376. [Google Scholar] [CrossRef] [PubMed]
- Kamya, P.Z.; Byrne, M.; Graba-Landry, A.; Dworjanyn, S.A. Near-future ocean acidification enhances the feeding rate and development of the herbivorous juveniles of the Crown of Thorns Starfish, Acanthaster planci. Coral Reefs 2016. [Google Scholar] [CrossRef]
- Benzie, J.; Black, K.; Moran, P.; Dixon, P. Small-Scale Dispersion of Eggs and Sperm of the Crown-of Thorns Starfish (Acanthaster planci) in a Shallow Coral Reef Habitat. Biol. Bull. 1994, 186, 153–167. [Google Scholar] [CrossRef]
- Uthicke, S.; Logan, M.; Liddy, M.; Francis, D.; Hardy, N.; Lamare, M. Climate change as an unexpected co-factor promoting coral eating seastar (Acanthaster planci) outbreaks. Sci. Rep. 2015, 5, 8402. [Google Scholar] [CrossRef] [PubMed]
- Wolanski, E.; Van Senden, D. Mixing of Burdekin river flood waters in the Great Barrier Reef. Mar. Freshw. Res. 1983, 34, 49–63. [Google Scholar] [CrossRef]
- Schroeder, T.; Devlin, M.J.; Brando, V.E.; Dekker, A.G.; Brodie, J.E.; Clementson, L.A.; McKinna, L. Inter-annual variability of wet season freshwater plume extent into the Great Barrier Reef lagoon based on satellite coastal ocean colour observations. Mar. Pollut. Bull. 2012, 65, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Sarantchova, O.L. Research into tolerance for the environment salinity in sea starfish Asterias rubens L. from populations of the White Sea and Barentz Sea. J. Exp. Mar. Biol. Ecol. 2001, 264, 15–28. [Google Scholar] [CrossRef]
- Casties, I.; Clemmesen, C.; Melzner, F.; Thomsen, J. Salinity dependence of recruitment success of the sea star Asterias rubens in the brackish western Baltic Sea. Helgol. Mar. Res. 2015, 69, 169–175. [Google Scholar] [CrossRef]
- Pia, T.S.; Johnson, T.; George, S.B. Salinity-induced morphological changes in Pisaster ochraceus (Echinodermata: Asteroidea) larvae. J. Plankton Res. 2012, 34, 590–601. [Google Scholar] [CrossRef]
- Strathmann, R.R. The feeding behavior of planktotrophic echinoderm larvae: mechanisms, regulation, and rates of suspension-feeding. J. Exp. Mar. Biol. Ecol. 1971, 6, 109–160. [Google Scholar] [CrossRef]
- George, S.B. Phenotypic plasticity in the larvae of Luidia foliolata (Echinodermata: Asteroidea). In Echinoderms through Time; David, B., Guille, A., Roux, M., Eds.; A.A. Balkema: Rotterdam, The Netherlands, 1994; pp. 297–307. [Google Scholar]
- George, S.B. Egg quality, larval growth and phenotypic plasticity in a forcipulate seastar. J. Exp. Mar. Biol. Ecol. 1999, 237, 203–224. [Google Scholar] [CrossRef]
- Sameoto, J.A.; Metaxas, A. Can salinity-induced mortality explain larval vertical distribution with respect to a halocline? Biol. Bull. 2008, 214, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Sameoto, J.A.; Metaxas, A. Interactive effects of haloclines and food patches on the vertical distribution of 3 species of temperate invertebrate larvae. J. Exp. Mar. Biol. Ecol. 2008, 367, 131–141. [Google Scholar] [CrossRef]
- Bashevkin, S.M.; Lee, D.; Driver, P.; Carrington, E.; George, S.B. Prior exposure to low salinity affects the vertical distribution of Pisaster ochraceus (Echinodermata: Asteroidea) larvae in haloclines. Mar. Ecol. Prog. Ser. 2016, 542, 123–140. [Google Scholar] [CrossRef]



| pH 8.1 | pH 7.6 | |||||||
|---|---|---|---|---|---|---|---|---|
| 27 | 29 | 31 | 34 | 27 | 29 | 31 | 34 | |
| 8.08 ± 0.02 | 8.07 ± 0.02 | 8.07 ± 0.02 | 8.07 ± 0.01 | 7.61 ± 0.01 | 7.61 ± 0.01 | 7.61 ± 0.01 | 7.61 ± 0.02 | |
| pHNIST | 8.09 ± 0.01 | 7.61 ± 0.00 | ||||||
| TA (μmol/kg) | 1968.3±12.0 | 2026.9±7.1 | 2166.4±12.9 | 2309.4±2.0 | 1968.3±12.0 | 2026.9±7.1 | 2166.4±12.9 | 2309.4±2.0 |
| pCO2 (ppm) | 492.4 | 493.1 | 514.3 | 529.9 | 1682.1 | 1696.2 | 1779.6 | 1851.0 |
| Variable | β | p-Value | Exp(β) |
|---|---|---|---|
| Salinity | 0.295 | >0.001 | 1.343 |
| Male/Female Cross | −0.146 | >0.001 | 0.864 |
| Time | 1.025 | >0.001 | 2.788 |
| Dependent Variable | Fixed Effects | df | F | p |
|---|---|---|---|---|
| Percent embryos hatched | Salinity | 3136 | 18.244 | <0.001 |
| Time | 1136 | 196.418 | <0.001 | |
| pH | 2136 | 1.094 | 0.298 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allen, J.D.; Schrage, K.R.; Foo, S.A.; Watson, S.-A.; Byrne, M. The Effects of Salinity and pH on Fertilization, Early Development, and Hatching in the Crown-of-Thorns Seastar. Diversity 2017, 9, 13. https://doi.org/10.3390/d9010013
Allen JD, Schrage KR, Foo SA, Watson S-A, Byrne M. The Effects of Salinity and pH on Fertilization, Early Development, and Hatching in the Crown-of-Thorns Seastar. Diversity. 2017; 9(1):13. https://doi.org/10.3390/d9010013
Chicago/Turabian StyleAllen, Jonathan D., Kharis R. Schrage, Shawna A. Foo, Sue-Ann Watson, and Maria Byrne. 2017. "The Effects of Salinity and pH on Fertilization, Early Development, and Hatching in the Crown-of-Thorns Seastar" Diversity 9, no. 1: 13. https://doi.org/10.3390/d9010013






