Species Richness, Taxonomic Distinctness and Environmental Influences on Euphausiid Zoogeography in the Indian Ocean
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Euphausiid Distributions
2.2. Measures of Diversity
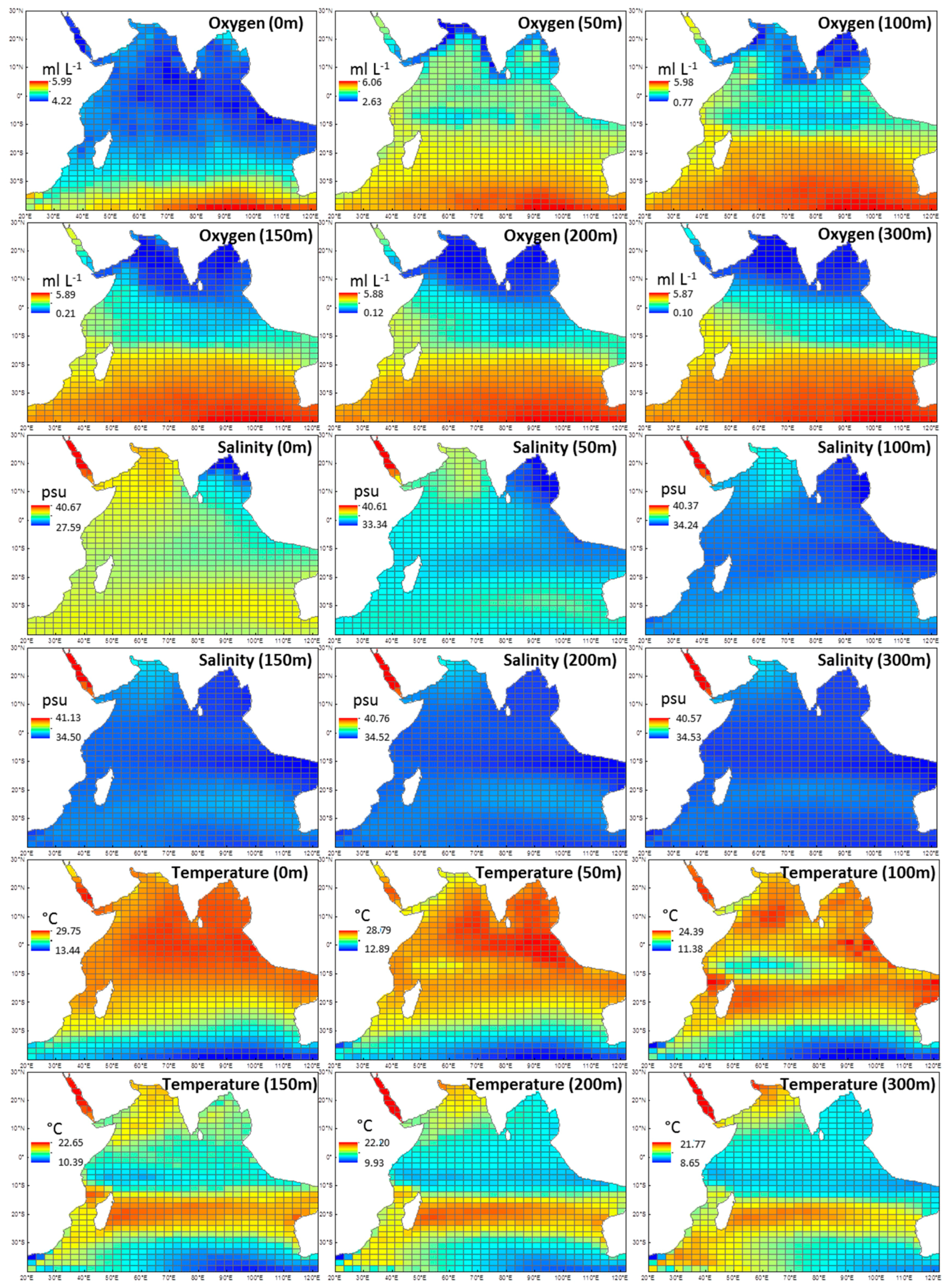
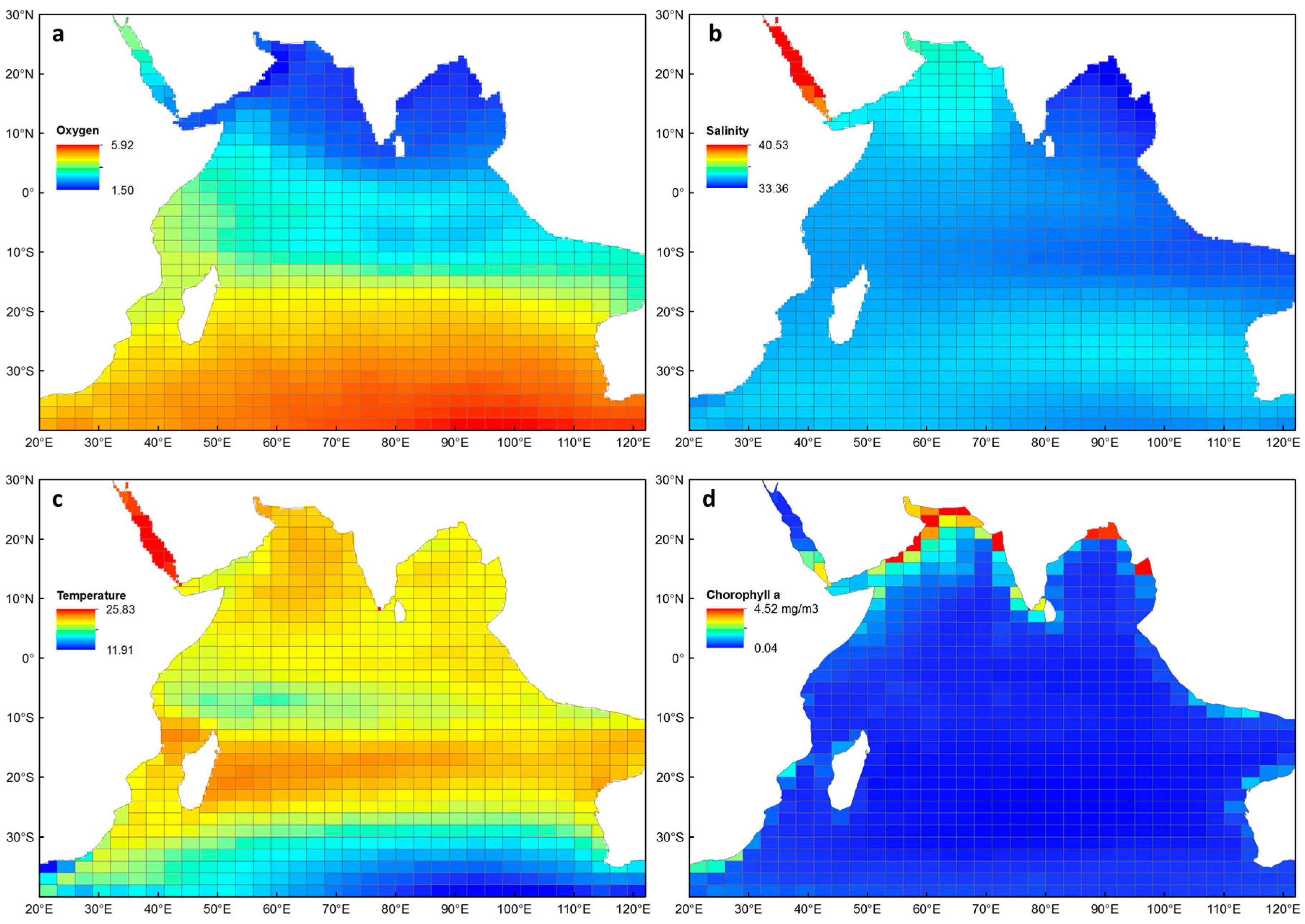
2.3. Environmental Explanatory Variables
2.4. Statistical Modelling
3. Results
3.1. Euphausiid Species Richness in the Indian Ocean
3.2. Taxonomic Distinctness of Euphausiids in the Indian Ocean
3.3. The Indian Ocean Environment
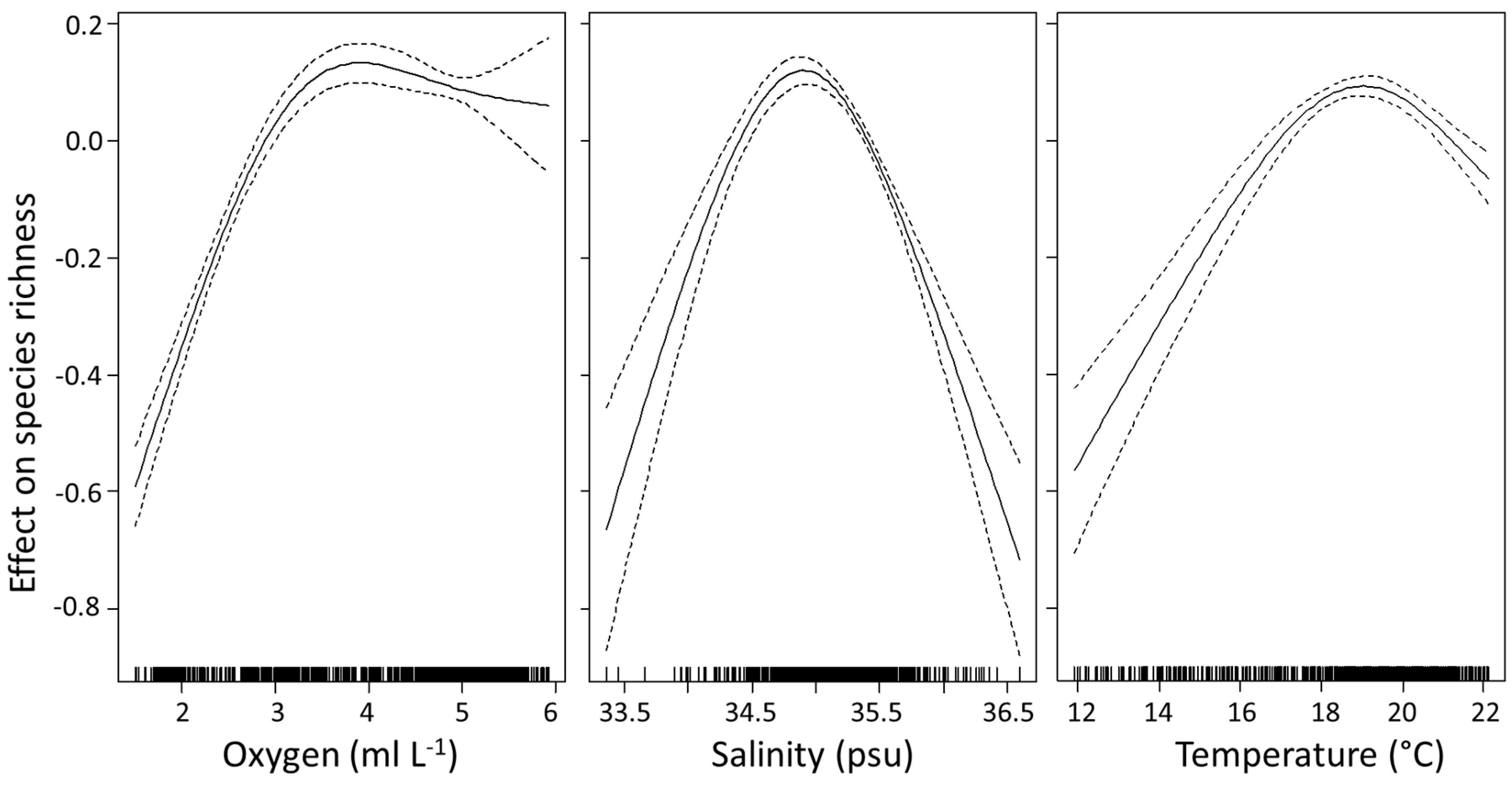
3.4. Environmental Explanations of Euphausiid Zoogeography
3.4.1. Species Richness
3.4.2. Average Taxonomic Distinctness
4. Discussion
5. Conclusion
Acknowledgements
Author Contributions
Conflicts of Interest
Appendix A
Appendix B
| Euphausiid Species | ||
| Bentheuphausia amblyops (4%) | Nematobrachion flexipes (86%) | Stylocheiron microphthalma (47%) |
| Euphausia brevis (71%) | Nematobrachion sexspinosum (44%) | Stylocheiron robustum (34%) |
| Euphausia diomedae (65%) | Nematoscelis atlantica (55%) | Stylocheiron suhmi (58%) |
| Euphausia fallax (3%) | Nematoscelis gracilis (65%) | Thysanoessa gregaria (36%) |
| Euphausia hemigibba (69%) | Nematoscelis megalops (30%) | Thysanopoda acutifrons (15%) |
| Euphausia longirostris (1%) | Nematoscelis microps (75%) | Thysanopoda aequalis (59%) |
| Euphausia lucens (14%) | Nematoscelis tenella (87%) | Thysanopoda astylata (22%) |
| Euphausia mutica (65%) | Nyctiphanes australis (1%) | Thysanopoda cornuta (8%) |
| Euphausia paragibba (38%) | Nyctiphanes capensis (1%) | Thysanopoda cristata (58%) |
| Euphausia pseudogibba (15%) | Pseudeuphausia latifrons (31%) | Thysanopoda egregia (6%) |
| Euphausia recurva (40%) | Stylocheiron abbreviatum (91%) | Thysanopoda microphthalma (<1%) |
| Euphausia sanzoi (19%) | Stylocheiron affine (89%) | Thysanopoda minyops (1%) |
| Euphausia sibogae (32%) | Stylocheiron armatum (5%) | Thysanopoda monocantha (60%) |
| Euphausia similis (54%) | Stylocheiron carinatum (95%) | Thysanopoda obtusifrons (54%) |
| Euphausia similisarmata (16%) | Stylocheiron elongatum (85%) | Thysanopoda orientalis (76%) |
| Euphausia spinifera (25%) | Stylocheiron indicum (1%) | Thysanopoda pectinata (86%) |
| Euphausia tenera (61%) | Stylocheiron insulare (3%) | Thysanopoda spinicaudata (1%) |
| Euphausia vallentini (1%) | Stylocheiron longicorne (95%) | Thysanopoda tricuspidata (63%) |
| Nematobrachion boopis (89%) | Stylocheiron maximum (91%) | |
References
- Brinton, E. The distribution of Pacific euphausiids. Bull. Scripps Inst. Oceanogr. 1962, 8, 51–269. [Google Scholar]
- Mauchline, J.; Fisher, L.R. The biology of euphausiids. In Advances in Marine Biology; Russell, F.S., Younge, M., Eds.; Academic Press: London, UK, 1969; Volume 7, pp. 1–54. [Google Scholar]
- Kawamura, A. A review of food of balaenopterid whales. Sci. Rep. Whales Res. Inst. 1980, 32, 155–197. [Google Scholar]
- Hipfner, M.J. Euphausiids in the diet of a North Pacific seabird: Annual and seasonal variation and the role of ocean climate. Mar. Ecol. Prog. Ser. 2009, 390, 277–289. [Google Scholar] [CrossRef]
- Itoh, T.; Kemps, H.; Totterdell, J. Diet of young southern bluefin tuna Thunnus maccoyii in the southwestern coastal waters of Australia in summer. Fish. Sci. 2011, 77, 337–344. [Google Scholar]
- Baker, A.C.; Boden, P.; Brinton, E. A Practical Guide to Euphausiids of the World.; Natural History Museum: London, UK, 1990. [Google Scholar]
- Brinton, E.; Ohman, M.D.; Townsend, A.W.; Knight, M.D.; Bridgeman, A.L. Euphausiids of the World Ocean World Biodiversity Database CD-ROM Series; Springer: Paris, France, 2000. [Google Scholar]
- Gibbons, M. Pelagic biogeography of the South Atlantic Ocean. Mar. Biol. 1997, 129, 757–768. [Google Scholar] [CrossRef]
- Brinton, E. Euphausiids of southeast Asian waters. In Scientific Results of Marine Investigations of the South China Sea and the Gulf of Thailand; Naga Report; University of California, Scripps Institution of Oceanography: La Jolla, CA, USA, 1975; Volume 4, pp. 1–287. [Google Scholar]
- Tittensor, D.P.; Mora, C.; Jetz, W.; Lotze, H.K.; Ricard, D.; Berghe, E.V.; Worm, B. Global patterns and predictors of marine biodiversity across taxa. Nature 2010, 466, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Letessier, T.B.; Cox, M.J.; Brierley, A.S. Drivers of variability in euphausiid species abundance throughout the Pacific Ocean. J. Plankton Res. 2011, 33, 1342–1357. [Google Scholar] [CrossRef]
- Letessier, T.B.; Cox, M.J.; Brierley, A.S. Drivers of euphausiid species abundance and numerical abundance in the Atlantic Ocean. Mar. Biol. 2009, 156, 2539–2553. [Google Scholar] [CrossRef]
- Brinton, E. Parameters relating to the distribution of planktonic organisms, especially euphausiids in the eastern tropical Pacific. Prog. Oceanogr. 1979, 8, 125–189. [Google Scholar] [CrossRef]
- Taki, K. Vertical distribution and diel migration of euphausiids from Oyashio Current to Kuroshio area off northeastern Japan. Plankton Benthos Res. 2008, 3, 27–35. [Google Scholar] [CrossRef]
- Sutton, A.L.; Beckley, L.E.; Holliday, D. Euphausiid assemblages in and around a developing anticyclonic Leeuwin Current eddy in the south-east Indian Ocean. J. R. Soc. West. Aust. 2015, 98, 9–18. [Google Scholar]
- Gibbons, M.; Barange, M.; Hutchings, L. Zoogeography and diversity of euphausiids around southern Africa. Mar. Biol. 1995, 123, 257–268. [Google Scholar] [CrossRef]
- Tarling, G.A.; Ward, P.; Sheader, M.; Williams, J.A.; Symon, C. Distribution patterns of macrozooplankton assemblages in the southwest Atlantic. Mar. Ecol. Prog. Ser. 1995, 120, 29–40. [Google Scholar] [CrossRef]
- Schott, F.A.; McCreary, J.P. The monsoon circulation in the Indian Ocean. Prog. Oceanogr. 2001, 51, 1–123. [Google Scholar] [CrossRef]
- Shankar, D.; Vinayachandran, P.N.; Unnikrishnan, A.S. The monsoon currents in the north Indian Ocean. Prog. Oceanogr. 2002, 52, 63–120. [Google Scholar] [CrossRef]
- Schott, F.A.; Xie, S.P.; McCreary, J.P. Indian Ocean circulation and climate variability. Rev. Geophys. 2009, 47. [Google Scholar] [CrossRef]
- Wiggert, J.D.; Murtugudde, R.G.; Christian, J.R. Annual ecosystem variability in the tropical Indian Ocean: Results of a coupled bio-physical ocean general circulation model. Deep-Sea Res. II 2006, 53, 644–676. [Google Scholar] [CrossRef]
- Naqvi, S.W.A.; Narvekar, P.V.; Desa, E. Coastal biogeochemical processes in the North Indian Ocean. In The Sea; Robinson, A., Brink, K., Eds.; Harvard University Press: Cambridge, MA, USA, 2006; Volume 14, pp. 723–780. [Google Scholar]
- Morrison, J.M.; Codispoti, L.A.; Smith, S.L.; Wishner, K.; Flagg, C.; Gardner, W.D.; Gaurin, S.; Naqvi, S.W.A.; Manghnani, V.; Prosperie, L.; Gundersen, J.S. The oxygen minimum zone in the Arabian Sea during 1995. Deep Sea Res. II 1999, 46, 1903–1931. [Google Scholar] [CrossRef]
- Naqvi, S.W.A.; Naik, H.; Jayakumar, A.; Pratihary, A.; Narvenkar, G.; Kurian, S.; Agnihotri, R.; Shailaja, M.S.; Narvekar, P.V. Seasonal anoxia over the western Indian continental shelf. In Indian Ocean Biogeochemical Processes and Ecological Variability; Wiggert, J.D., Hood, R.R., Naqvi, S.W.A., Smith, S.L., Brink, K.H., Eds.; American Geophysical Union: Washington, DC, USA, 2009; pp. 333–345. [Google Scholar]
- Gordon, A.L.; Fine, R.A. Pathways of water between the Pacific and Indian oceans in the Indonesian seas. Nature 1996, 379, 146–149. [Google Scholar] [CrossRef]
- Wijffels, S.; Meyers, G. An intersection of oceanic waveguides: Variability in the Indonesian Throughflow region. J. Phys. Oceanogr. 2004, 34, 1232–1253. [Google Scholar] [CrossRef]
- McCreary, J.P.; Miyama, T.; Furue, R.; Jensen, T.; Kang, H.W.; Bang, B.; Qu, T. Interactions between the Indonesian throughflow and circulations in the Indian and Pacific Oceans. Prog. Oceanogr. 2007, 75, 70–114. [Google Scholar] [CrossRef]
- Xu, J. Change of Indonesian Throughflow outflow in response to East Asian monsoon and ENSO activities since the last glacial. Sci. China Ser. D Earth Sci. 2014, 57, 791–801. [Google Scholar] [CrossRef]
- Meyers, G.; Bailey, R.J.; Worby, A.P. Geostrophic transport of Indonesian throughflow. Deep Sea Res. I 1995, 42, 1163–1174. [Google Scholar] [CrossRef]
- Domingues, C.M.; Maltrud, M.E.; Wijffels, S.E.; Church, J.A.; Tomczak, M. Simulated Lagrangian pathways between the Leeuwin Current System and the upper-ocean circulation of the southeast Indian Ocean. Deep Sea Res. II 2007, 54, 797–817. [Google Scholar] [CrossRef]
- Brinton, E.; Gopalakrishnan, K. The distribution of Indian Ocean euphausiids. In Biology of the Indian Ocean; Zeitzschel, B., Gerlach, S.A., Eds.; Springer Berlin Heidelberg: London, UK, 1973; pp. 357–382. [Google Scholar]
- Wilson, S.G.; Meekan, M.G.; Carleton, J.H.; Stewart, T.C.; Knott, B. Distribution, abundance and reproductive biology of Pseudeuphausia latifrons and other euphausiids on the southern North West Shelf, Western Australia. Mar. Biol. 2003, 142, 369–379. [Google Scholar] [CrossRef]
- Gallienne, C.P.; Conway, D.V.P.; Robinson, J.; Naya, N.; William, J.S.; Lynch, T.; Meunier, S. Epipelagic mesozooplankton distribution and abundance over the Mascarene Plateau and Basin, south-western Indian Ocean. J. Mar. Biol. Assoc. UK 2004, 84, 1–8. [Google Scholar] [CrossRef]
- Jayalakshmi, K.J.; Jasmine, P.; Muraleedharan, K.R.; Prabhakaran, M.P.; Habeebrehman, H.; Jacob, J.; Achuthankutty, C.T. Aggregation of Euphausia sibogae during Summer Monsoon along the Southwest Coast of India. J. Mar. Biol. 2011, 2011, 1–12. [Google Scholar] [CrossRef]
- Brinton, E. Vertical migration and avoidance capability of euphausiids in the California Current. Limnol. Oceanogr. 1967, 12, 451–483. [Google Scholar] [CrossRef]
- Wiebe, P.H.; Boyd, S.H.; Davis, B.M.; Cox, J.L. Avoidance of towed nets by the euphausiid Nematoscelis megalops. Fish. Bull. 1982, 80, 75–91. [Google Scholar]
- Gotelli, N.J.; Colwell, R.K. Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol. Lett. 2001, 4, 379–391. [Google Scholar] [CrossRef]
- Warwick, R.M.; Clarke, K.R. New “biodiversity” measures reveal a decrease in taxonomic distinctness with increasing stress. Mar. Ecol. Prog. Ser. 1995, 129, 301–305. [Google Scholar] [CrossRef]
- Tolimieri, N.; Anderson, M.J. Taxonomic distinctness of demersal fishes of the California Current: moving beyond simple measures of diversity for marine ecosystem-based management. PLoS ONE 2010, 5, e10653. [Google Scholar] [CrossRef] [PubMed]
- Tweedley, J.R.; Warwick, R.M.; Valesini, F.J.; Platell, M.E.; Potter, I.C. The use of benthic macroinvertebrates to establish a benchmark for evaluating the environmental quality of microtidal, temperate southern hemisphere estuaries. Mar. Pollut. Bull. 2012, 64, 1210–1221. [Google Scholar] [CrossRef] [PubMed]
- Clarke, K.R.; Warwick, R.M. A taxonomic distinctness index and its statistical properties. J. App. Ecol. 1998, 35, 523–531. [Google Scholar] [CrossRef]
- Clarke, K.R.; Warwick, R.M. Changes in Marine Communities: An Approach to Statistical Analysis and Interpretation; PRIMER-E Ltd.: Plymouth, UK, 2001. [Google Scholar]
- Clarke, K.R.; Gorley, R.N. PRIMER v7: User Manual/Tutorial; PRIMER-E Ltd.: Plymouth, UK, 2015. [Google Scholar]
- Clarke, K.R.; Warwick, R.M. The taxonomic distinctness measure of biodiversity: Weighting of step lengths between hierarchical levels. Mar. Ecol. Prog. Ser. 1999, 184, 21–29. [Google Scholar] [CrossRef]
- Taniguchi, A. Mysids and euphausiids in the eastern Indian Ocean with particular reference to invasion of species from the Banda Sea. J. Mar. Biol. Assoc. India 1974, 16, 349–357. [Google Scholar]
- Cassanova, B. Evolution spatiale et structurale des peuplements d’euphausiaces de l’Antarctique au Golfe d’Aden. Sci. Mar. 1980, 44, 377–394. [Google Scholar]
- Nair, S.R.; Nair, V.R.; Achuthankutty, C.T.; Madhupratap, M. Zooplankton composition and diversity in western Bay of Bengal. J. Plankton Res. 1981, 3, 493–508. [Google Scholar] [CrossRef]
- Mathew, K.J. The ecology of Euphausiacea along the southwest coast of India. J. Mar. Biol. Assoc. India 1985, 27, 138–157. [Google Scholar]
- Silas, E.G.; Mathew, K.J. Spatial distribution of Euphausiacea (Crustacea) in the southeastern Arabian Sea. J. Mar. Biol. Assoc. India 1986, 28, 1–21. [Google Scholar]
- Fatima, M. Euphausiids of Somalian waters and Gulf of Aden collected in S.W. Monsoon season. Pak. J. Sci. Ind. Res. 1987, 30, 935–937. [Google Scholar]
- Hirota, Y. Vertical distribution of euphausiids in the Western Pacific Ocean and the Eastern Indian Ocean. Bull. Jpn. Sea Reg. Fish. Res. Lab. 1987, 37, 175–224. [Google Scholar]
- Hitchcock, G.L.; Lane, P.; Smith, S.; Luo, J.; Ortner, P.B. Zooplankton spatial distribution on coastal waters of the northern Arabian Sea, August, 1995. Deep Sea Res. II 2002, 49, 2403–2423. [Google Scholar] [CrossRef]
- Mathew, K.J.; Sivan, G.; Krishnakumar, P.K.; Kuriakose, S. Euphausiids of the West Coast of India; Central Marine Fisheries Research Institute: Cochin, India, 2003; p. 149. [Google Scholar]
- Holliday, D.; Beckley, L.E.; Weller, E.; Sutton, A.L. Natural variability of macro-zooplankton and larval fishes off the Kimberley, north-western Australia: Preliminary findings. J. R. Soc. West. Aust. 2011, 94, 85–99. [Google Scholar]
- Sutton, A.L.; Beckley, L.E. Influence of the Leeuwin Current on the epipelagic euphausiid assemblages of the south-east Indian Ocean. Hydrobiologia 2016, 779, 193–207. [Google Scholar] [CrossRef]
- Sutton, A.L.; Beckley, L.E. Euphausiid assemblages of the oceanographically complex north-west marine bioregion of Australia. Mar. Fresh. Res. 2017. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Austin, M.P. Spatial prediction of species distribution: An interface between ecological theory and statistical modelling. Ecol. Model. 2002, 157, 101–118. [Google Scholar] [CrossRef]
- Wood, S.N. Generalised Additive Models, An Introduction with R, 2nd ed.; Chapman and Hall/CRC: Boca Raton, FL, USA, 2006; 392p. [Google Scholar]
- Zuur, A.F. A Beginner’s Guide to Generalised Additive Models with R; Highland Statistics Ltd.: Newburgh, UK, 2012; p. 194. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Multimodel inference—Understanding AIC and BIC in model selection. Sociol. Methods Res. 2004, 33, 261–304. [Google Scholar] [CrossRef]
- Swartzman, G.; Hickey, B.; Kosro, P.M.; Wilson, C. Poleward and equatorward currents in the Pacific Eastern Boundary Current in summer 1995 and 1998 and their relationship to the distribution of euphausiids. Deep Sea Res. II 2005, 52, 73–88. [Google Scholar] [CrossRef]
- Nicol, S. Krill, currents, and sea ice: Euphausia superba and its changing environment. Bioscience 2006, 56, 111. [Google Scholar] [CrossRef]
- De Grave, S.; Pentcheff, N.D.; Ahyong, S.T.; Chan, T.-Y.; Crandall, K.A.; Dworschak, P.C.; Felder, D.L.; Feldmann, R.M.; Fransen, C.H.J.M.; Goulding, L.Y.D.; et al. A classification of living and fossil genera of decapod crustaceans. Raffles Bull. Zool. 2009, 21, 1–109. [Google Scholar]
- Bucklin, A.; Wiebe, P.H.; Smolenack, A.B.M.; Copley, N.J.; Beaudet, J.G.; Bonner, K.G.; Farber-Lorda, J.; Pierson, J.J. DNA barcodes for species identification of euphausiids (Euphausiacea, Crustacea). J. Plankton Res. 2007, 29, 483–493. [Google Scholar]
- Hood, R.R.; Beckley, L.E.; Wiggert, J.D. Biogeochemical and ecological impacts of boundary currents in the Indian Ocean. Prog. Oceanogr. 2017. [Google Scholar] [CrossRef]
- Paulmier, A.; Ruiz-Pino, D. Oxygen minimum zones (OMZs) in the modern ocean. Prog. Oceanogr. 2009, 80, 113–128. [Google Scholar]
- Maas, A.E.; Frazer, S.H.; Outram, D.M.; Seibel, B.A.; Wishner, K.E. Fine-scale vertical distribution of macroplankton and micronekton in the Eastern Tropical North Pacific in association with an oxygen minimum zone. J. Plankton Res. 2014, 36, 1557–1575. [Google Scholar]
- Wishner, K.F.; Ashjian, C.J.; Gelfman, C.; Gowing, M.M.; Kann, L.; Levin, A.; Mullineaux, L.S.; Saltzman, J. Pelagic and benthic ecology of the lower interface of the Eastern Tropical Pacific oxygen minimum zone. Deep Sea Res. I 1995, 42, 93–115. [Google Scholar]
- Antezana, T. Species-specific patterns of diel migration into the oxygen minimum zone by euphausiids in the Humboldt Current ecosystem. Prog. Oceanogr. 2009, 83, 228–236. [Google Scholar]
- Vinayachandran, P.N.; Kurian, J. Hydrographic observations and model simulations of the Bay of Bengal freshwater plume. Deep Sea Res. I 2007, 54. [Google Scholar] [CrossRef]
- Willig, M.R.; Kaufman, D.M.; Stevens, R.D. Latitudinal gradients of biodiversity: Pattern, process, scale, and synthesis. Ann. Rev. Ecol. Evol. Syst. 2003, 34, 273–309. [Google Scholar]
- Hillebrand, H. On the generality of the latitudinal diversity gradient. Am. Nat. 2004, 163, 192–211. [Google Scholar] [PubMed]
- Fuhrman, J.A.; Steele, J.A.; Hewson, I.; Schwalbach, M.S.; Brown, M.V.; Green, J.L.; Brown, J.H. A latitudinal diversity gradient in planktonic marine bacteria. Proc. Natl. Acad. Sci. USA 2008, 105, 7774–7778. [Google Scholar] [PubMed]
- Sameoto, D.; Guglielmi, L.; Lewis, M.K. Day/night vertical distribution of euphausiids in the eastern tropical Pacific. Mar. Biol. 1987, 96, 235–245. [Google Scholar] [CrossRef]
- Vialard, J.; Duvel, J-P.; McPhaden, M.; Bouruet-Aubertot, P.; Ward, B.; Key, E.; Bourras, D.; Weller, R.; Minnett, P.; Weill, A.; et al. Cirene: Air sea interactions in the Seychelles-Chagos thermocline ridge region. Bull. Am. Met. Soc. 2009, 90, 45–61. [Google Scholar] [CrossRef]
- Irigoien, X.; Huisman, J.; Harris, R.P. Global biodiversity patterns of marine phytoplankton and zooplankton. Nature 2004, 429, 863–867. [Google Scholar] [CrossRef] [PubMed]

| Published Source | Indian Ocean Region | Sampling Net; Mesh Size | Depth Range (m) | # Samples |
|---|---|---|---|---|
| [45] Taniguchi 1974 | Indo-Australian Basin | Indian Ocean standard net * (1 m2); 0.33 mm | 0–2976 | 164 |
| Norpac net (50 cm and 56cm diameter) | ||||
| [46] Cassanova 1980 | Western Indian Ocean | Indian Ocean standard net * (1 m2); 0.33 mm | 0–4000 | 88 |
| 200 cm long net (44–48.5 cm diameter); 0.30 mm | ||||
| [47] Nair et al. 1981 | Bay of Bengal | Indian Ocean standard net * (1 m2); 0.33 mm | 0–200 | 22 |
| [48] Mathew 1985 | Arabian Sea | Indian Ocean standard net * (1 m2); 0.33 mm | 0–150 | 182 |
| [49] Silas and Mathew 1986 | Arabian Sea | Indian Ocean standard net * (1 m2); 0.33 mm | 0–1300 | 312 |
| Isaacs Kidd Mid-water Trawl | ||||
| [50] Fatima 1987 | Somalia, Gulf of Aden | Indian Ocean standard net * (1m2); 0.33 mm | Unknown | 9 |
| [51] Hirota 1987 | Eastern Indian Ocean | Multi-depth sampling (MTD) net (56 cm diameter); 0.10 mm, 0.33 mm, 0.68 mm | 0–1000 | 52 |
| [16] Gibbons et al. 1995 # | Southern Africa | Several net types | Various | Various |
| [7] Brinton et al. 2000 # | Whole basin | Indian Ocean standard net * (1 m2); 0.33 mm | 0–200 | 1231 |
| Several net types | Various | |||
| [52] Hitchcock et al. 2002 | Arabian Sea | MOCNESS (1 m2); 0.153 mm | 0–1200 | 6 |
| [53] Mathew et al. 2003 | Arabian Sea | Bongo net (60 cm diameter); 0.33 mm | 0–150 | 493 |
| [32] Wilson et al. 2003 | Eastern Indian Ocean, NW shelf Australia | Light traps | 0–75 | 426 |
| [33] Galliene et al. 2004 | Southwest Indian Ocean | Conical net (40 cm diameter); 0.125 mm | 0–50 | 36 |
| [54] Holliday et al. 2011 | Kimberley, eastern Indian Ocean | Bongo net (50 cm diameter); 0.35 mm | 0–150 | 72 |
| [34] Jayalakshmi et al. 2011 | Arabian Sea | Multiple Plankton Net Sampler (0.25 m2); 0.20 mm | Mixed layer/thermocline | 9 |
| [15] Sutton et al. 2015 | Southeast Indian Ocean | EZ net (1 m2); 0.335 mm | 0–200 | 36 |
| [55] Sutton and Beckley 2016 | Southeast Indian Ocean | Bongo net (50 cm diameter); 0.355 mm | 0–150 | 26 |
| [56] Sutton and Beckley 2017 | Indo-Australian Basin | Bongo net (50 cm diameter); 0.355 mm | 0–150 | 108 |
| Variable | Unit | Source | Time Period | Resolution/Average |
|---|---|---|---|---|
| Average temperature (0–300 m) | °C | www.marine.csiro.au/atlas/ | 1950–2009 | 0.5° × 0.5°/annually |
| Average salinity (0–300 m) | psu | www.marine.csiro.au/atlas/ | 1950–2009 | 0.5° × 0.5°/annually |
| Average oxygen (0–300 m) | ml L−1 | www.marine.csiro.au/atlas/ | 1950–2009 | 0.5° × 0.5°/annually |
| Average surface chlorophyll a | mg m−3 | https://oceancolor.gsfc.nasa.gov | 2002–2013 | 1°/annually |
| GAM Model | % Deviance Explained | Adjusted r2 | p Value | n |
|---|---|---|---|---|
| Species richness ~ s(oxygen) + s(salinity) + s(temperature) + chlorophyll a | 78.3 | 0.783 | <0.001 | 701 |
| AveTD ~ s(oxygen) + s(salinity) + s(chlorophyll a) | 20.4 | 0.194 | <0.001 | 701 |
| Variable | EDF | Chi-Squared | p Value | Difference in % Deviance Explained |
|---|---|---|---|---|
| Oxygen | 2.6 | 468.9 | <0.001 | 13.4 |
| Salinity | 2.8 | 140.3 | <0.001 | 4.4 |
| Temperature | 2.8 | 144.4 | <0.001 | 4.7 |
| Chlorophyll a | 1.0 | <0.001 | 0.8 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sutton, A.L.; Beckley, L.E. Species Richness, Taxonomic Distinctness and Environmental Influences on Euphausiid Zoogeography in the Indian Ocean. Diversity 2017, 9, 23. https://doi.org/10.3390/d9020023
Sutton AL, Beckley LE. Species Richness, Taxonomic Distinctness and Environmental Influences on Euphausiid Zoogeography in the Indian Ocean. Diversity. 2017; 9(2):23. https://doi.org/10.3390/d9020023
Chicago/Turabian StyleSutton, Alicia L., and Lynnath E. Beckley. 2017. "Species Richness, Taxonomic Distinctness and Environmental Influences on Euphausiid Zoogeography in the Indian Ocean" Diversity 9, no. 2: 23. https://doi.org/10.3390/d9020023





