Introduction
The requirement of continuous monitoring for environmental and medical applications has been taken place in a main interest of developing a variety of miniaturised sensors for chemical and biological analytes in aqueous solutions [
1,
2,
3,
4,
5,
6,
7]. In combination with the possibility of using silicon planar technology, some miniaturised potentiometric sensors have been developed and are now available [
8]. The advantage of such sensors is their process compatibility with integrated circuit technology. Furthermore, they are appropriate for on-line monitoring, where small analyte volumes are needed. However, these miniaturised sensors still suffer from the drawback of an integrated reference electrode, also fabricated by the same technology as the existing sensor chip. For instance, a microfabricated liquid junction Ag/AgCl reference electrode and a reference field-effect transistor are proposed in ref. [
9,
10]. Although there exists a great interest of miniaturised reference electrodes that are compatible to current silicon technology, no long-term stable reference electrode has been prepared so far, possessing comparable features as for conventional macroscopic reference electrodes [
11,
12,
13].
A first step to realise a thin-film pH sensor together with an integrated thick-film Ag/AgCl reference electrode is demonstrated in this paper. Therefore, the reference electrode has been deposited on a silicon chip and coupled with a capacitive pH sensor. Thick-film technique was used to fabricate the reference electrode, because it is well-proved for industrial mass fabrication [
14]. In the presented application, no liquid-junction was used to get a simple fabrication of the reference electrode for special applications in biological and environmental applications with a constant chloride concentration. The capacitive thin-film pH sensor can be fabricated by a single “three step” process (thermal oxidation, pulsed laser deposition, electron beam evaporation). For the realisation of the hybrid sensor chip, no photolithographic process steps are necessary and thus, the sensor chip can be prepared in a easy and cheap way.
Experimental
The Ag/AgCl reference electrode was fabricated by means of standard thick-film technique. Therefore, a semi-automatic screen printer (Model TF 100; MPM, Franklin MA) was utilised. An Ag/AgCl ink (Ercon R-414) was printed through a patterned stencil in bands of (2-3) mm * (30-40) mm on a <100> silicon wafer. The resulting printed thick film electrodes were cured for 60 min at 120°C and the wafer was seperated in samples of 10 mm * 20 mm.
P-doped silicon <100>-wafers (Fa. Wacker-Chemitronic), with a thickness of 1 mm and a specific resistance of 1-2 Ωcm were used to fabricate the pH sensor. The wafer was seperated in chips of (10*10) mm
2. After a typical RCA purification process, described for example in [
15], 30 nm SiO
2 was thermally grown at 1000°C for one hour on top of the silicon substate (oxidation oven, Fa. Tempress). As pH-sensitive layer, 50 nm of Al
2O
3 was brought up on the SiO
2 by means of pulsed laser deposition (PLD) technique at a temperature of 940°C. A 200 nm thick aluminium layer as rear contact was deposited by means of electron beam evaporation. All experimental details are described elsewhere [
16,
17,
18].
The reference electrode and pH sensor were commonly bonded on a printed circuit board. The pH sensor was contacted on the rear side and the screen-printed electrode on the Ag/AgCl strip. Both chips were finally encapsulated with an epoxy compound (EPO-TEK 87-GT, Fa. Polytec).
The electrochemical characterisation of the pH sensor/Ag/AgCl reference hybrid chip occured with the computer-controlled impedance measuring system IM5d (Fa. Zahner Elektrik). Measurements were made in a two-electrode configuration. Following different combinations of pH sensor and reference electrode were used:
- -
thin-film pH sensor and thick-film Ag/AgCl reference electrodes (SPE1, SPE2, SPE3),
- -
thin-film pH sensor and conventional macroscopic Ag/AgCl reference electrode (Fa. Metrohm, Switzerland).
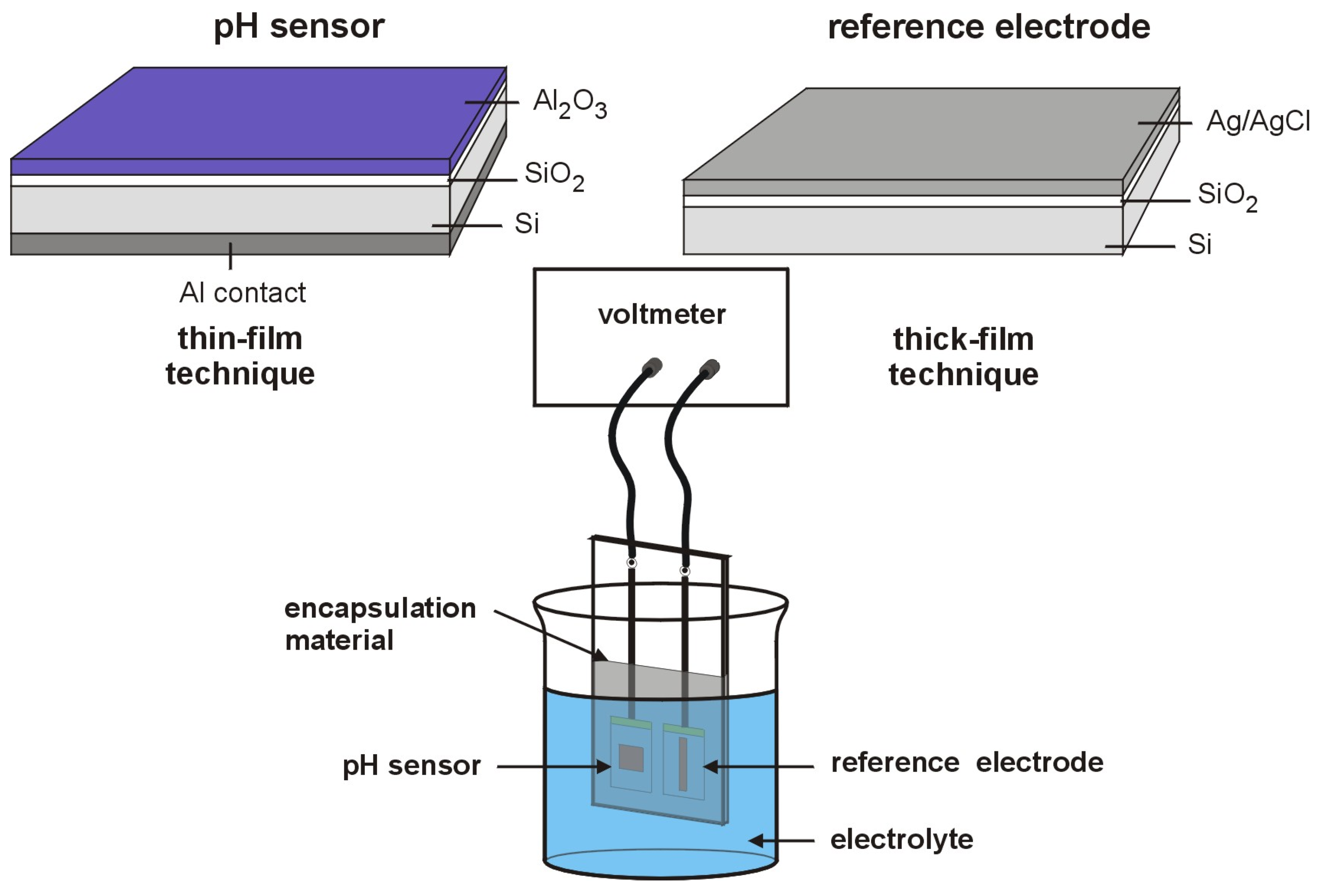
The measurement set-up and a schematic picture of both, reference electrode and the pH sensor, are demonstrated in
Fig. 1. For all measurements technical buffer solutions in the range of pH 4 to pH 9 with a constant chloride concentration of about 0.05 mM (Titrisol, Merck) were used as electrolyte. The measurements were carried out in a dark Faraday cage at room temperature. When not in use the thin-film pH sensor was stored in de-ionized water, whereas the thick-film reference electrode was stored in dry atmosphere.
Figure 1.
Schematic picture of the measurement set-up and the electrodes used. The pH sensor was fabricated by means of thin-film technique and the reference electrode by thick-film technique. Sensor and reference electrode were bonded on a common printed circuit board.
Figure 1.
Schematic picture of the measurement set-up and the electrodes used. The pH sensor was fabricated by means of thin-film technique and the reference electrode by thick-film technique. Sensor and reference electrode were bonded on a common printed circuit board.
For the C/V (capacitance/voltage) measurements, the d.c. voltage was swept from -1.5 V to 1.7 V in steps of 100 mV and an a.c. voltage was superposed with a frequency of 120 Hz and a signal amplitude of 20 mV. Depending on the change of the pH value, the position of the C/V curve shifts along the voltage axis. In the ConCap (constant capacitance) mode, by setting the capacitance at a fixed value (60% of the maximum capacitance), the voltage shift which results from changing the pH of the electrolyte can be directly recorded (decreased voltage value by increased H+-ion concentration). The ConCap measurements were carried out at the same frequency and an a.c. voltage amplitude of 20 mV. Measurements were started at pH 9 and the voltage was then recorded 5 minutes for each pH value. The solution was changed stepwise from pH 9 to pH 4 and backwards to pH 9. Before each concentration step, the pH sensor and reference electrode were rinsed with de-ionized water. To obtain the calibration curve of the pH sensor, the measured voltage values after 5 minutes were plotted versus the corresponding pH values.
Electrochemical impedance measurements of the thin-film pH sensor were performed towards a conventional macroscopic Ag/AgCl reference electrode and the thick-film Ag/AgCl reference electrode in a frequency range between 100 mHz and 2 MHz to study an eventual influence of the reference electrode´s impedance on the pH measurements. The investigations were carried out with Titrisol buffer, pH 7, and a constant chloride-ion concentration.
Results and Discussion
Fig. 2 presents typical C/V curves of the thin-film pH sensor in buffer pH 7, measured with a conventional Ag/AgCl reference electrode and the screen-printed thick-film reference electrodes at a frequency of 120 Hz. The electrochemical properties of the pH sensor are described in detail in [
19]. The first C/V curve was recorded with a conventional macroscopic Ag/AgCl reference electrode (I). Then, the pH sensor was characterised with the screen-printed thick-film reference electrodes (SPE1, SPE2, SPE3 ➔ II) and at last, a further C/V measurement was performed with the same conventional Ag/AgCl reference electrode (I) to exclude an eventual time-depending shift of the C/V curve. As can be seen, there is a comparable C/V-curve behaviour of the pH sensor according to the theory for the investigated type of reference electrode. The maximal capacitance is 32 nF, measured with the conventional Ag/AgCl reference electrode (first and last measurement) and the screen-printed thick-film electrodes SPE1, SPE2 and SPE3, respectively. The type of the reference electrode has an influence on the flat-band voltage of the C/V curve. Using a screen-printed thick-film reference electrode instead of a conventional Ag/AgCl reference electrode effects a shift of the C/V curve along the voltage axis of about 200 mV. However, the C/V-curve behaviour – serving as measuring signal – will not be interfered. Hence, the hybrid sensor chip can be applied for potentiometric investigations.
Figure 2.
C/V-curve behaviour of the thin-film pH-sensor versus various thick-film reference electrodes (SPE1, SPE2, SPE3) and a conventional macroscopic Ag/AgCl reference, respectively.
Figure 2.
C/V-curve behaviour of the thin-film pH-sensor versus various thick-film reference electrodes (SPE1, SPE2, SPE3) and a conventional macroscopic Ag/AgCl reference, respectively.
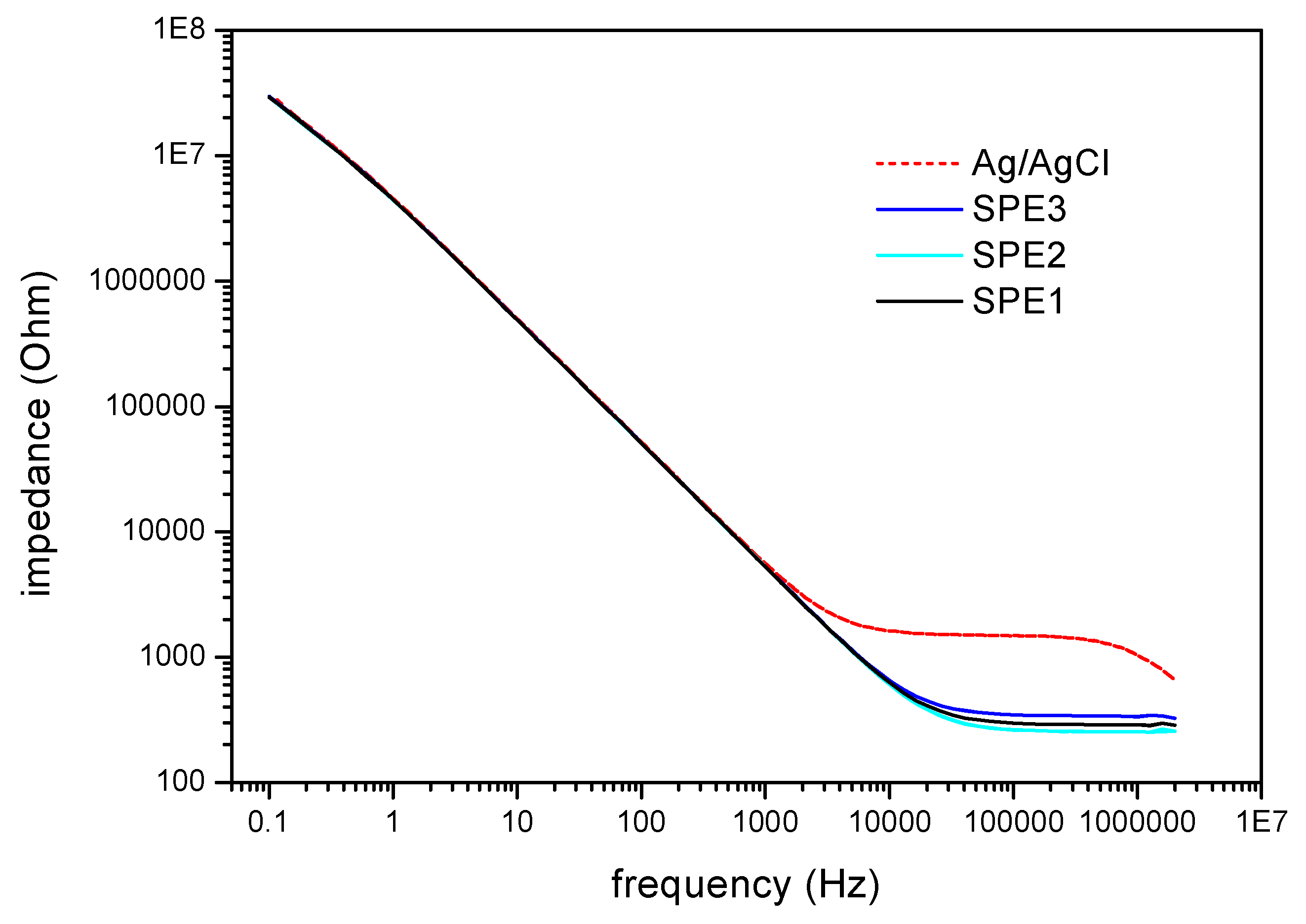
Fig. 3 depicts typical impedance measurements of the hybrid sensor chip. Nearly identical impedances for the thin-film pH sensor towards the investigated reference electrodes in the frequency range from 100 mHz to 1 kHz were observed. At frequencies greater than 10 kHz, the ohmic resistance of the respective reference appears. It amounts about 1.5 kΩ for the convential Ag/AgCl reference electrode and about 0.3 kΩ for the screen-printed thick-film reference electrodes. The values are calculated at a frequency of 100 kHz. The larger resistance of the conventional reference electrode is due to the additional resistance of the diaphragm. At a frequency of 120 Hz, used for C/V- and ConCap measurements, no effect of the reference electrode to the impedance signal exists.
Figure 3.
Impedance measurements of the thin-film pH sensor using different reference electrodes (conventional macroscopic Ag/AgCl reference and screen-printed thick-film electrodes). At a frequency of 120 Hz, used for the C/V- and ConCap measurements, no effect of the reference electrode´s impedance appears.
Figure 3.
Impedance measurements of the thin-film pH sensor using different reference electrodes (conventional macroscopic Ag/AgCl reference and screen-printed thick-film electrodes). At a frequency of 120 Hz, used for the C/V- and ConCap measurements, no effect of the reference electrode´s impedance appears.
An influence of the chloride-ion concentration to the screen-printed thick-film electrode is expected, because no inner electrolyte and no membrane seperates the Ag/AgCl paste from the electrolyte buffer used for measurements [
20]. Therefore, for the ConCap measurements, Titrisol buffer with a constant chloride concentration of about 0.05 M between pH 4 and pH 9 was utilised.
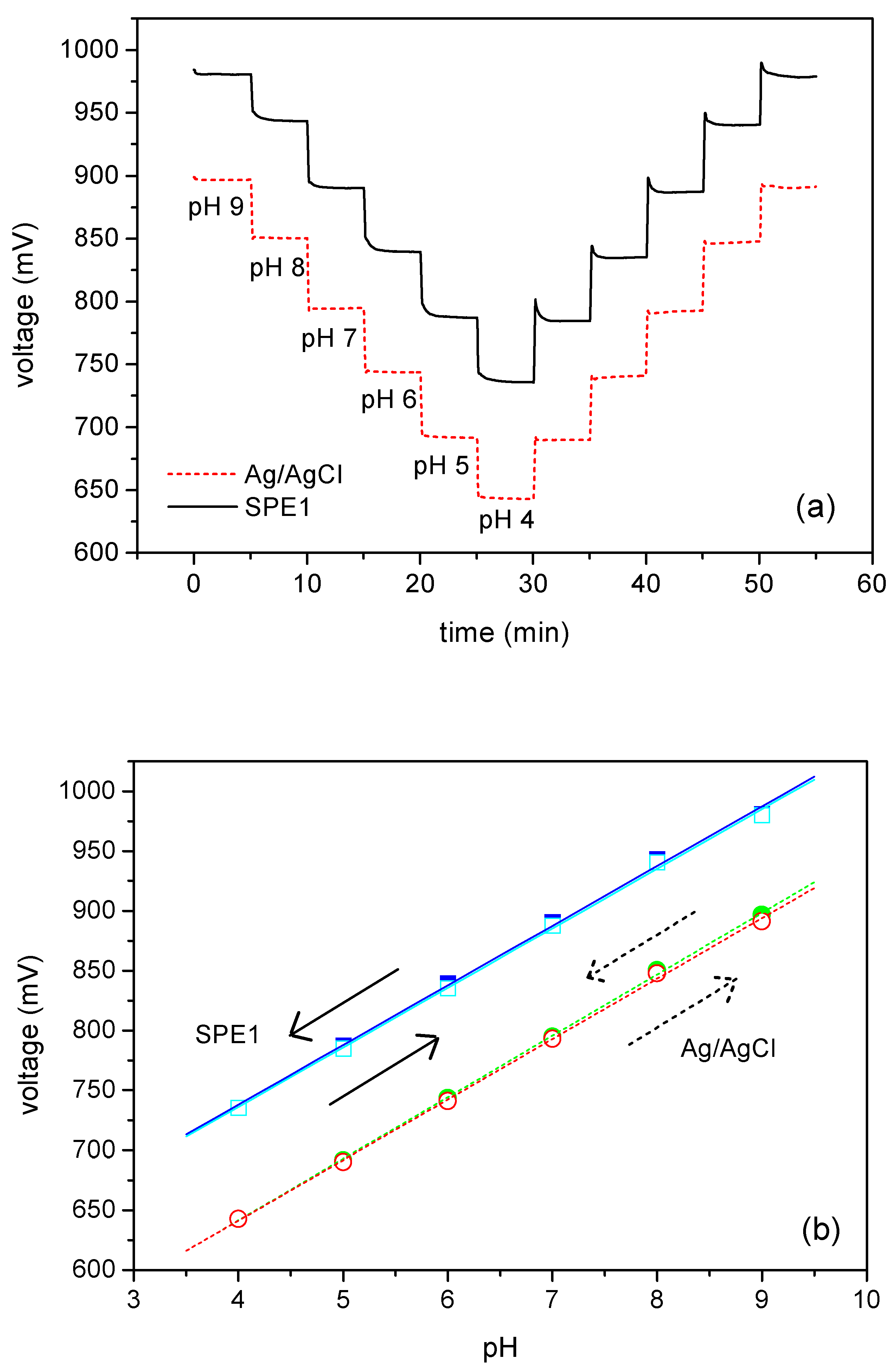
Fig. 4(a) exemplarily presents two ConCap measurements of the pH sensor, one measured with a screen-printed thick-film reference electrode (full line) and the other measured versus a conventional macroscopic Ag/AgCl reference electrode (dotted line) at a frequency of 120 Hz. The screen-printed thick-film electrode (SPE1) was bonded on the same printed circuit board as the pH sensor yielding the hybrid thin-film/thick-film sensor chip. The measurements, carried out with a screen-printed thick-film electrode and a conventional Ag/AgCl reference electrode show reproducible potentials for each pH value with short response times of smaller than one minute for both directions of changing the pH. The shift of the potential between the two ConCap cycles is due to the shift of flat-band voltage of the C/V curve explained in
Fig. 2. The smaller deviation in the case of the ConCap measurement (about 95 mV) in comparison to the C/V measurement (about 200 mV) can be verified by the additional chloride-ion concentration of about 0.05 M.
Figure 4.
ConCap measurements were carried out in electrolyte buffers between pH 4 and pH 9 with a constant chloride-ion concentration of 0.05 mM (a). The calibration curves demonstrate a good agreement in the pH sensitivity when utilising the screen-printed thick-film reference electrode (full line) and the conventional Ag/AgCl reference electrode (dotted line), respectively (b). The arrows mark the direction of the pH cycle.
Figure 4.
ConCap measurements were carried out in electrolyte buffers between pH 4 and pH 9 with a constant chloride-ion concentration of 0.05 mM (a). The calibration curves demonstrate a good agreement in the pH sensitivity when utilising the screen-printed thick-film reference electrode (full line) and the conventional Ag/AgCl reference electrode (dotted line), respectively (b). The arrows mark the direction of the pH cycle.
In
Fig. 4(b), the respective calibration curves are presented. As demonstrated in the calibration curves for both directions of changing pH (pH 9 ➔ pH 4; pH 4 ➔ pH 9) and both reference electrode types (conventional macroscopic Ag/AgCl reference and screen-printed thick-film electrode), a linear sensor response was observed. For the measurement carried out with the screen-printed thick-film electrode, the slope of the curve, i.e. the average sensitivity of the pH sensor amounts about 50 mV/pH independent of the titration direction (pH 9 ➔ pH 4; pH 4 ➔ pH 9). The ConCap measurement performed with the conventional reference electrode results in a sensitivity of 51 mV/pH (pH 9 ➔ pH 4; pH 4 ➔ pH 9). In both cases, the pH sensitivities show a good agreement. The difference of potential at pH 9 at the begin and at the end of the measurement cycle, i.e. the hysteresis, was about 2 mV (SPE1) and 5 mV (conventional Ag/AgCl electrode), respectively.
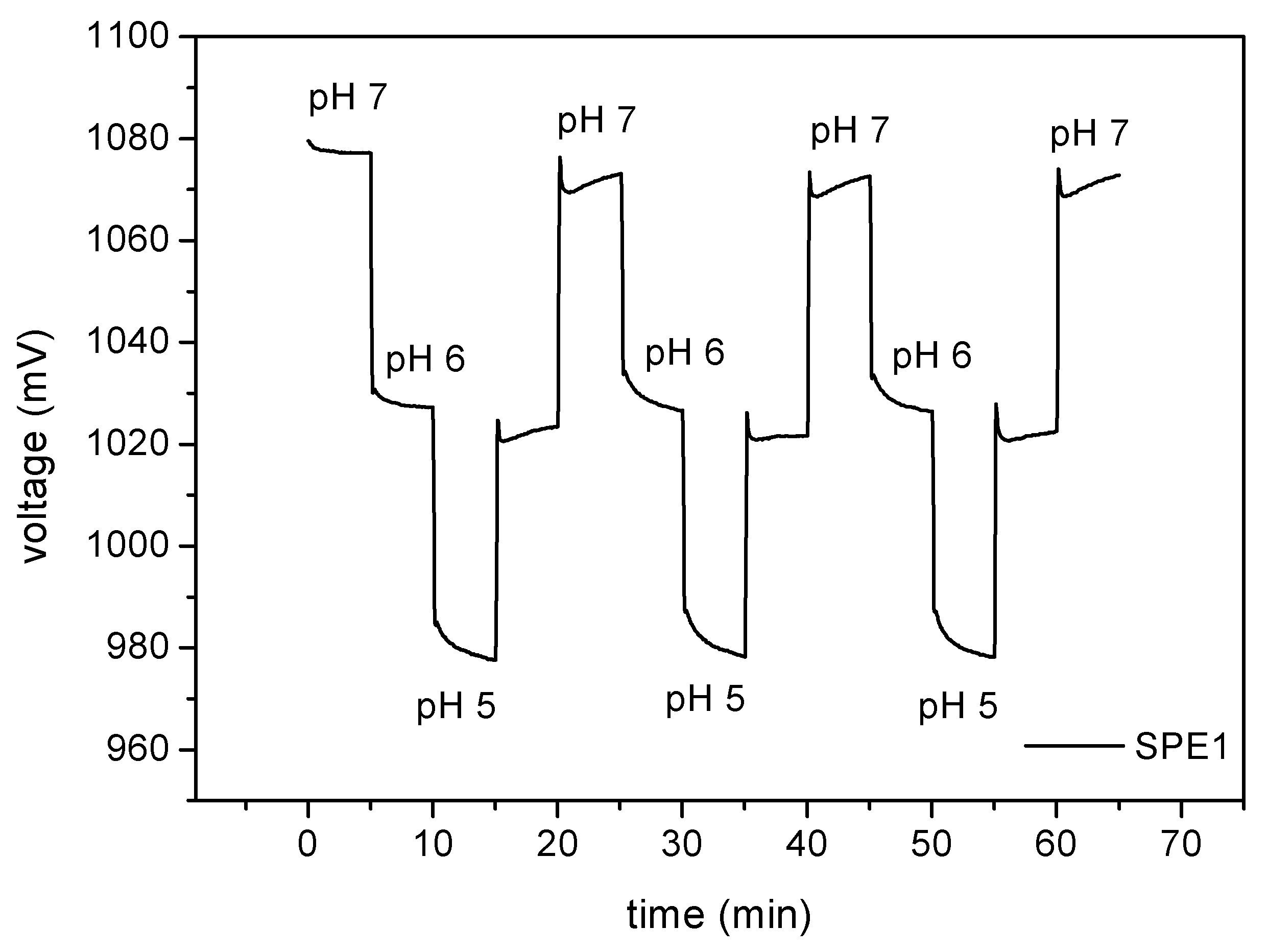
ConCap measurements between pH 7 and pH 5 were performed with the hybrid thin-film/thick-film sensor chip for investigating the reproducibility of the potentials after each measurement cycle. Therefore, the buffer solution was changed from pH 7 over pH 6 to pH 5 and
vice versa for three times. In
Fig. 5, a typical ConCap measurement for testing the reproducibility is examplarily illustrated. The maximal deviation between the measured voltage values for each pH amounts less than 5 mV. As can be seen, the potentials after each cycle are highly reproducible for each pH value.
Figure 5.
Repeated cycling of the pH between pH 5 and pH 7 for studying the reproducibility of the developed hybrid sensor chip. The maximal deviation between the measured voltage values for each pH is less than 5 mV.
Figure 5.
Repeated cycling of the pH between pH 5 and pH 7 for studying the reproducibility of the developed hybrid sensor chip. The maximal deviation between the measured voltage values for each pH is less than 5 mV.
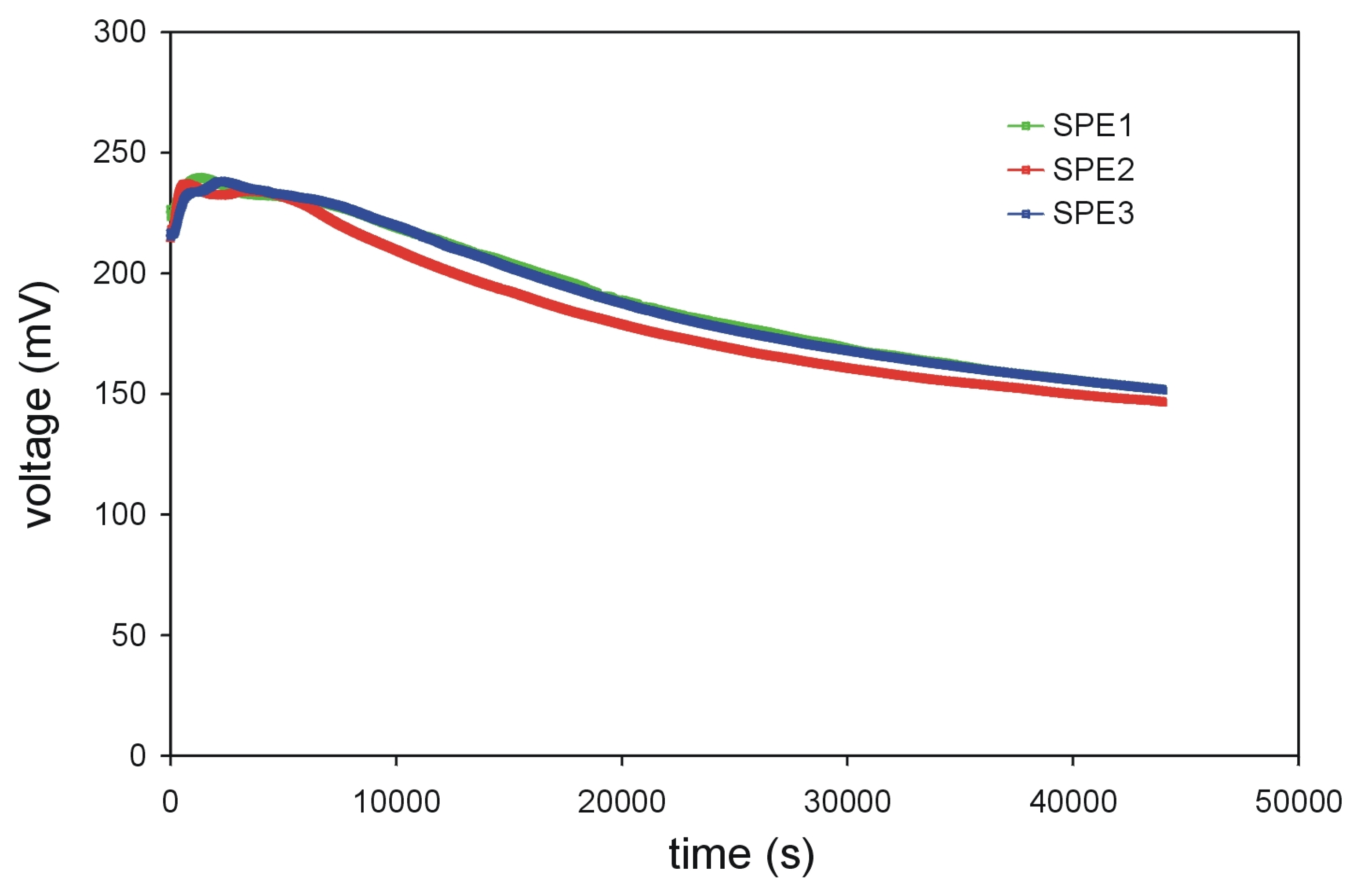
For investigating the time-dependent potential-shift of the screen-printed thick-film electrodes, the potential between a conventional Ag/AgCl reference electrode and the three screen-printed thick-film reference electrodes was continuously measured for more than ten hours (
Fig. 6). Previous measurements of the conventional Ag/AgCl reference electrode
versus a double-junction reference electrode have indicated a minimal drift of the Ag/AgCl reference electrode of less than 0.1 mV/hour. The drift of the screen-printed thick-film reference electrodes SPE1, SPE2 and SPE3 was about 60-70 mV in 12 hours. The resulting average drift rate is about 5 mV/hour. The worser drift rate in comparison to the macroscopic Ag/AgCl reference electrode is probably due to the missing liquid junction (including a constant inner electrode buffer). However, further investigations are necessary to prove this behavior in detail.
Figure 6.
Measurement of the potential between a conventional Ag/AgCl reference electrode and the screen-printed thick-film reference electrodes (SPE1, SPE2, SPE3).
Figure 6.
Measurement of the potential between a conventional Ag/AgCl reference electrode and the screen-printed thick-film reference electrodes (SPE1, SPE2, SPE3).
Conclusions
A pH sensor, fabricated by means of thin-film technique and a thick-film fabricated reference electrode were integrated on a common printed circuit board. For testing the suitability of the fabricated thin-film/thick-film sensor hybrid, C/V- and ConCap measurements were performed. Compared with measurements, carried out with a conventional Ag/AgCl reference electrode, an ideal C/V-curve behaviour could be achieved for the sensor hybrid. The difference in the flat-band voltage of the C/V curve between the two types of reference electrodes is due to the difference of the standard potentials between these reference electrodes. Impedance measurements in the frequency range between 100 mHz and 2 MHz were performed with the pH sensor and the different reference electrodes. No reference electrode-dependent impedance was viewable at a frequency of 120 Hz, used for C/V- and ConCap measurements. Sensitivity studies for pH, carried out with the thin-film/thick-film sensor hybrid showed results, which are highly comparable to those obtained with a single pH sensor when employing a conventional Ag/AgCl electrode. The calibration curve demonstrates a linear relationship between potential and pH value. The average pH sensitivity is about 50 mV/pH in the concentration range from pH 4 to pH 9, independent of the titration direction. The hybrid sensor chip shows a high reproducibility. To investigate the stability of the fabricated reference electrodes, potentiometric drift measurements versus a conventional Ag/AgCl reference electrode were performed for more than ten hours. The average drift rate of the thick-film reference electrode is about 5 mV/hour and should decrease for longer measuring periods. To overcome the disturbing effect of chloride ions, the C/V- and ConCap measurements were carried out with buffer, keeping the concentration of chloride ions constant.
The characterisation of the thin-film pH/thick-film reference hybrid chip could demonstrate that this system is well-suited for pH measurements. Further investigations are necessary to improve the voltage drift of the screen-printed thick-film reference electrode. A possible approach is the emloyment of membranes, covering the Ag/AgCl paste of the thick-film reference electrode, to protect the reference electrode from a fast leaching out of this chloride-containing paste [
21]. In the next step, we plan to integrate the thick-film reference electrode directly on the pH sensor chip. Then, a further miniaturisation of the hybrid thin-film/thick-film sensor chip, which can be easily tailored for e.g., a “flow injection”-type sensor, should be possible.