A Carbon Nanotube-based Sensor for CO2 Monitoring
Abstract
:Introduction
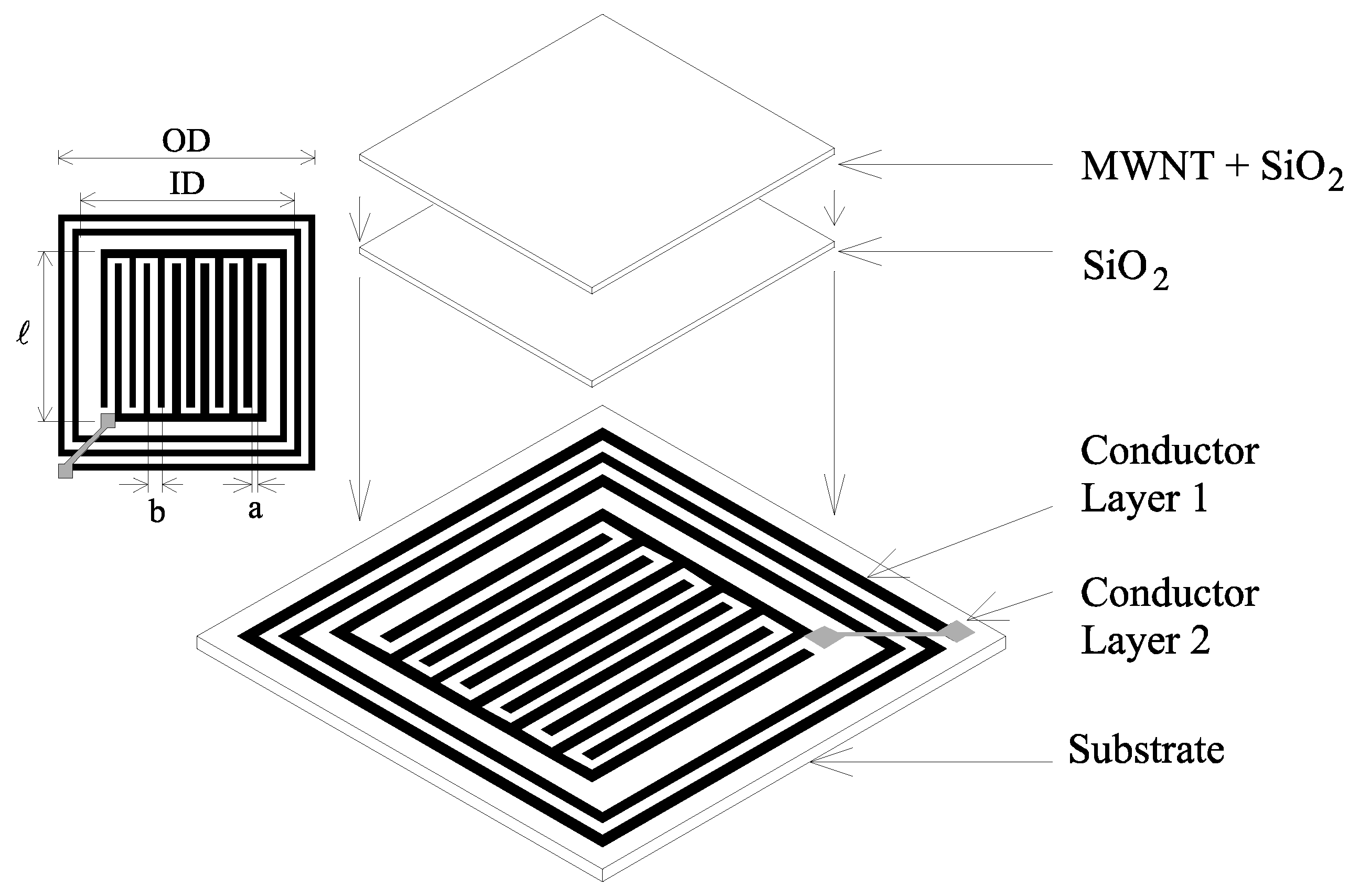



Experimental
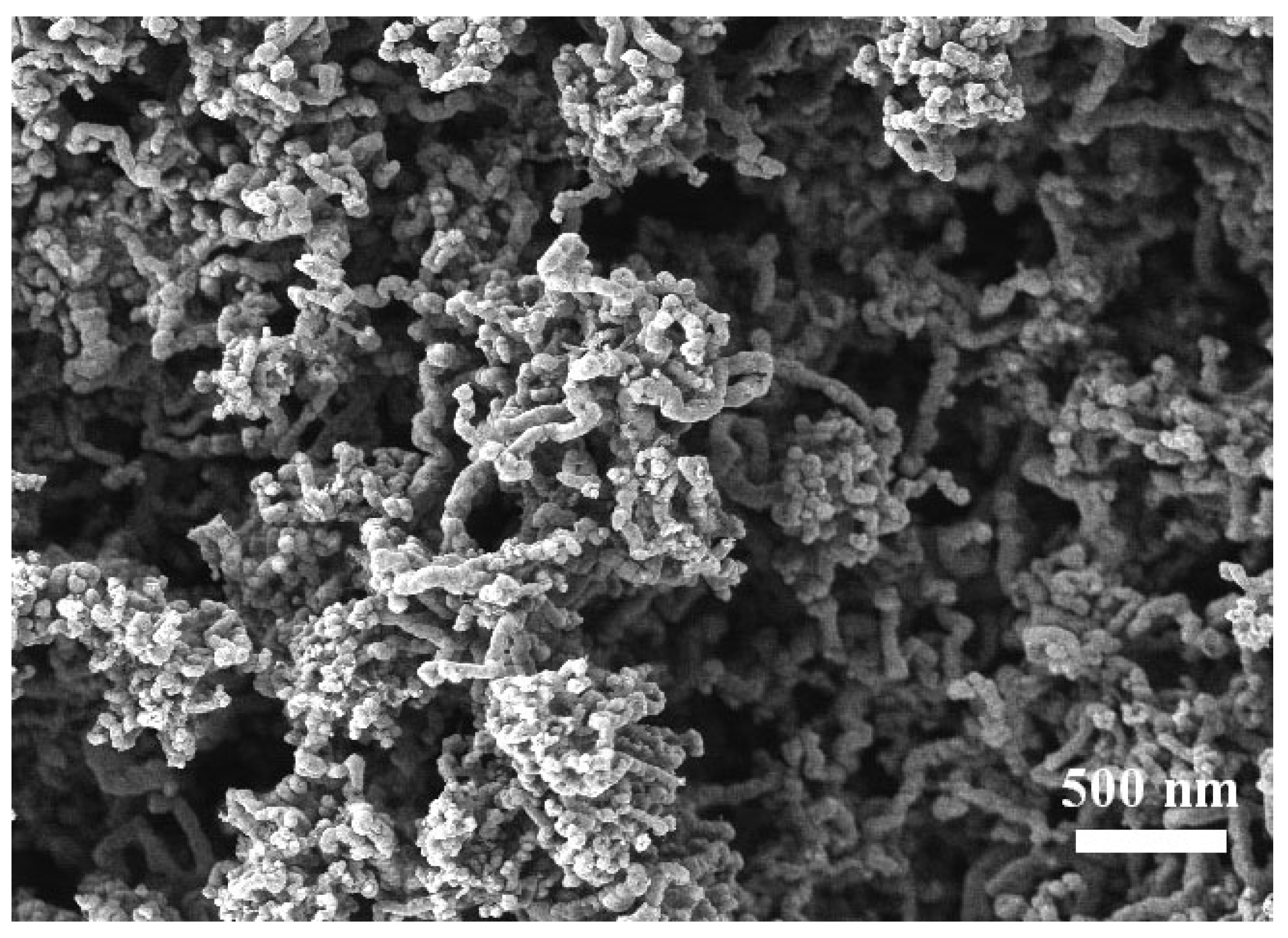
Preparation of nanotubes
Sensor Fabrication
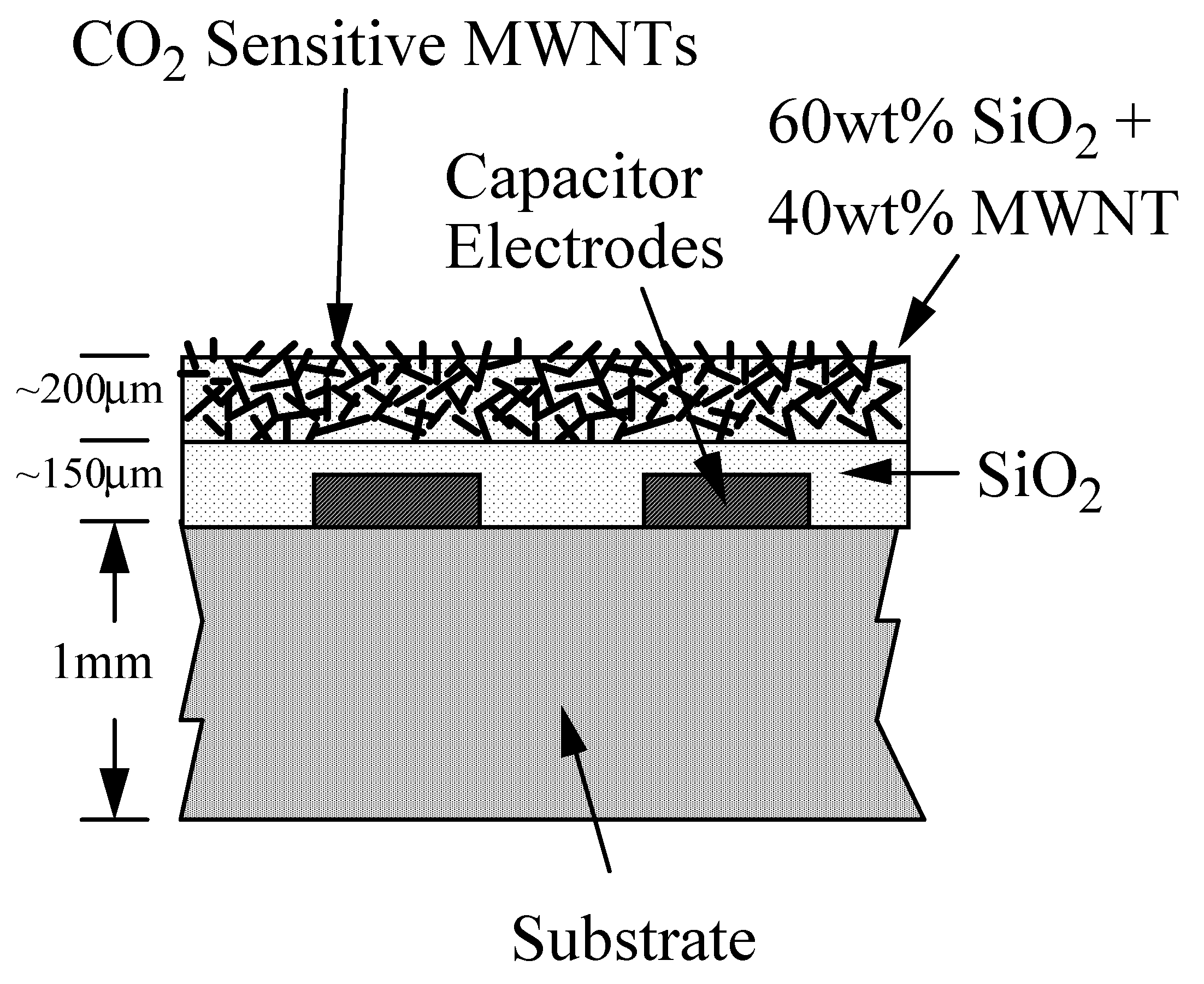
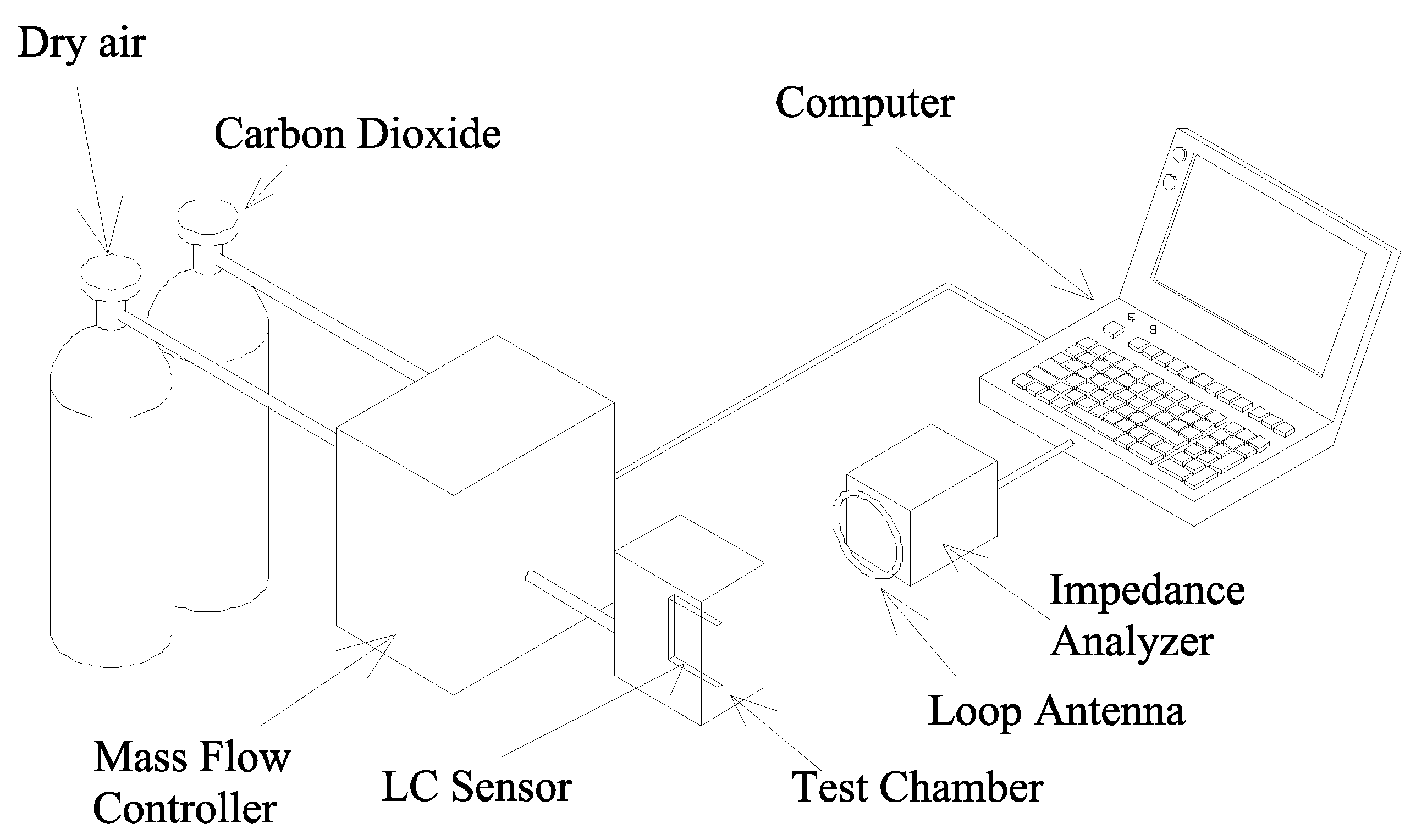
Experimental Setup for Sensing CO2
Results and Discussion
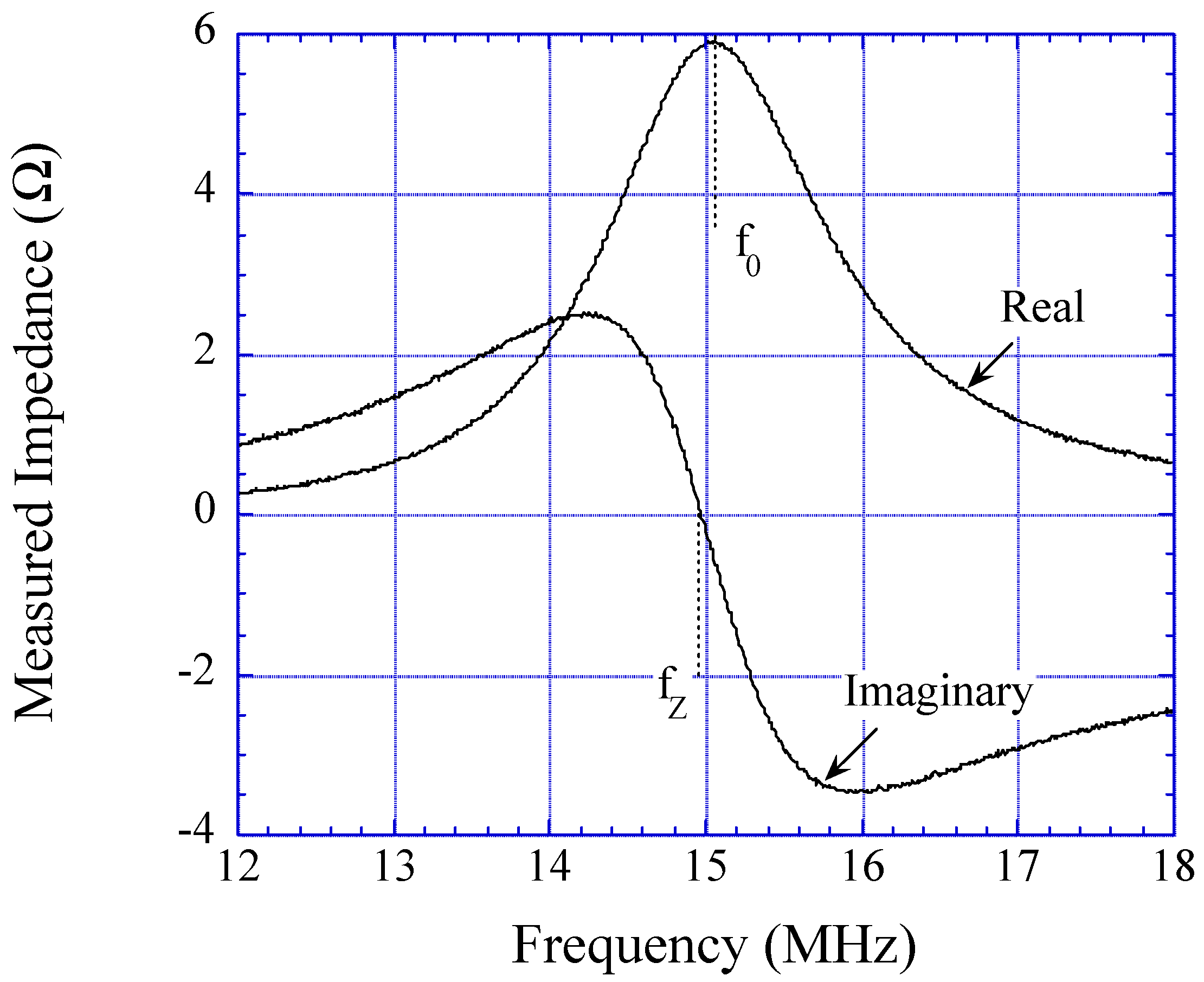
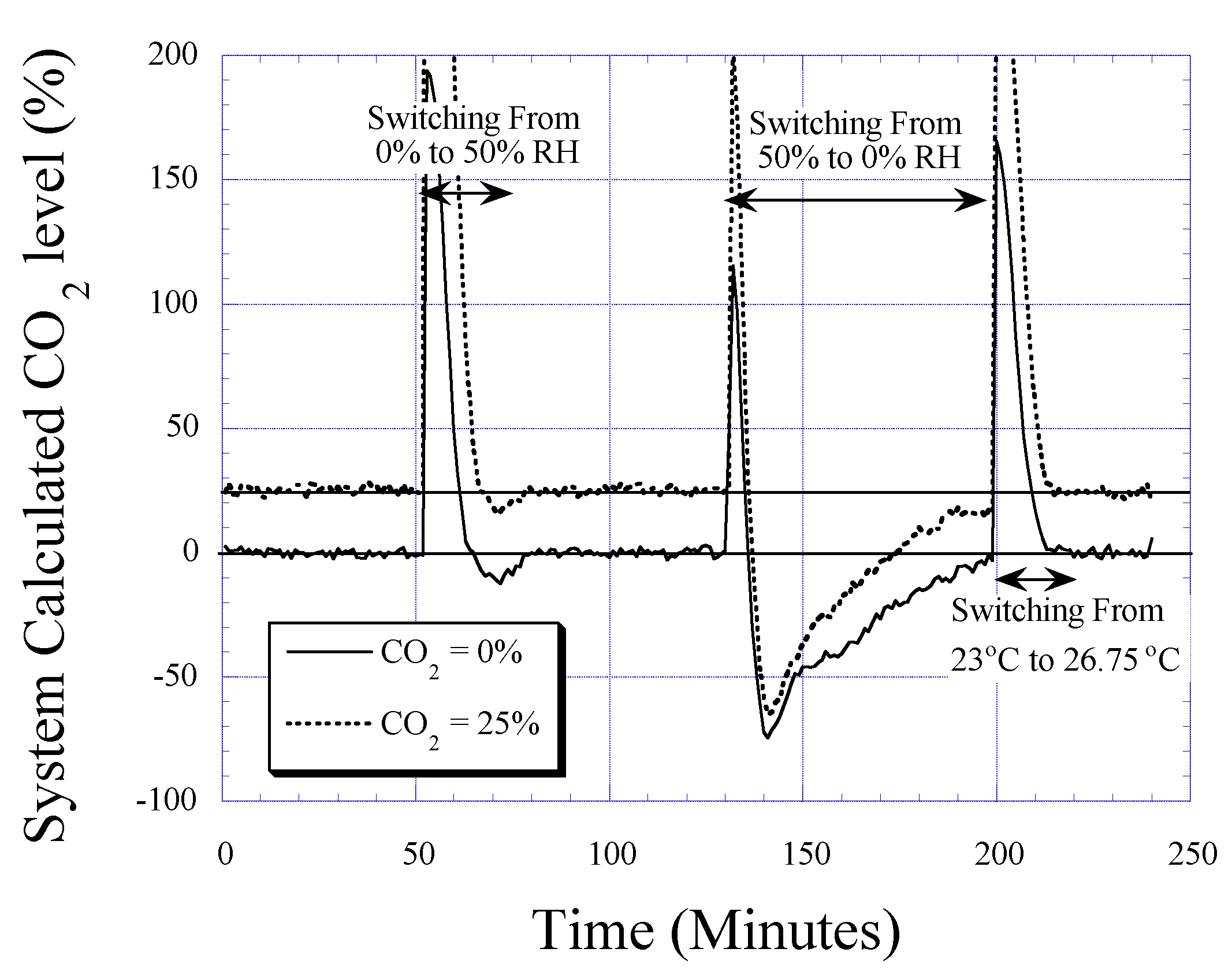
CO2 Detection
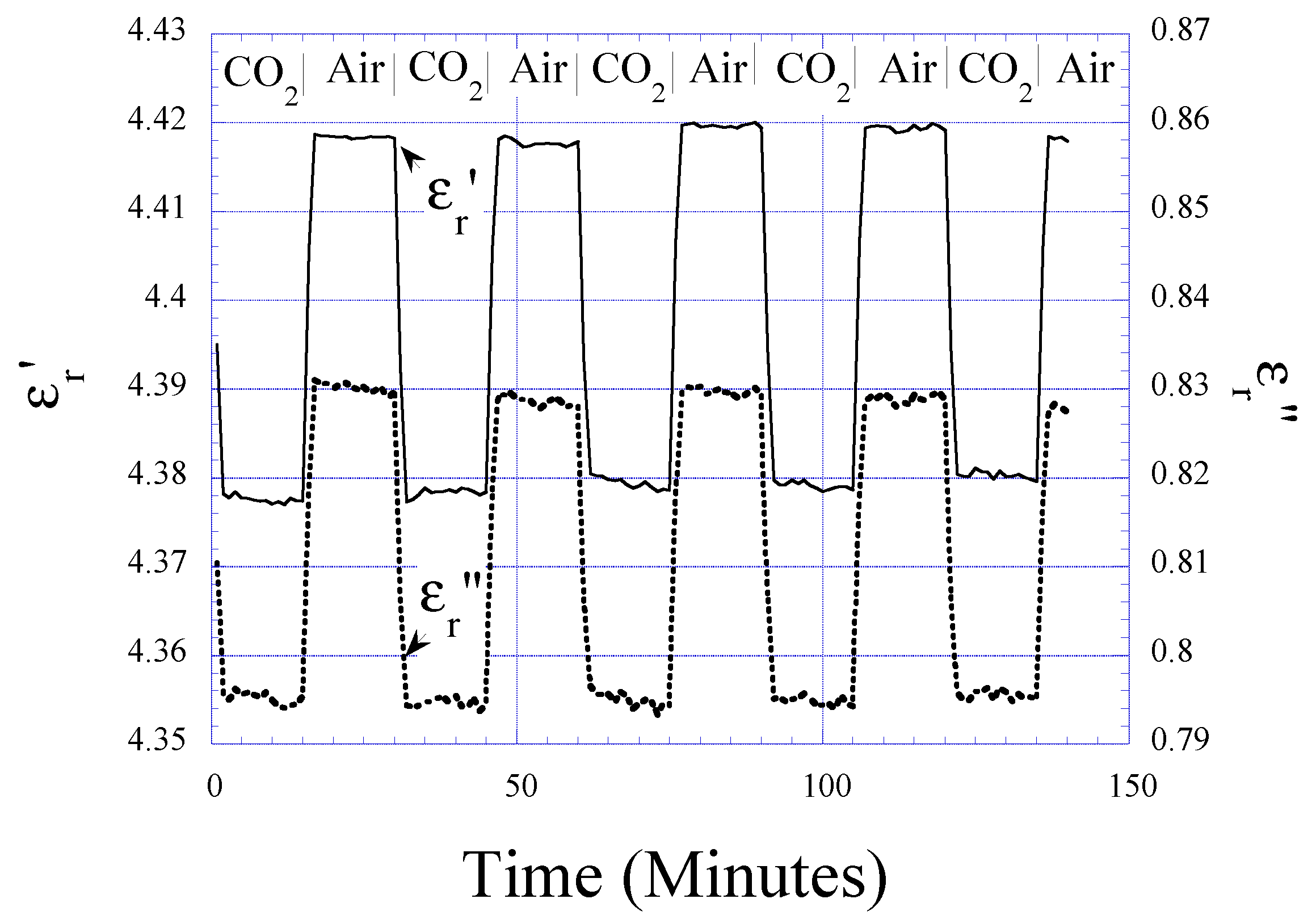
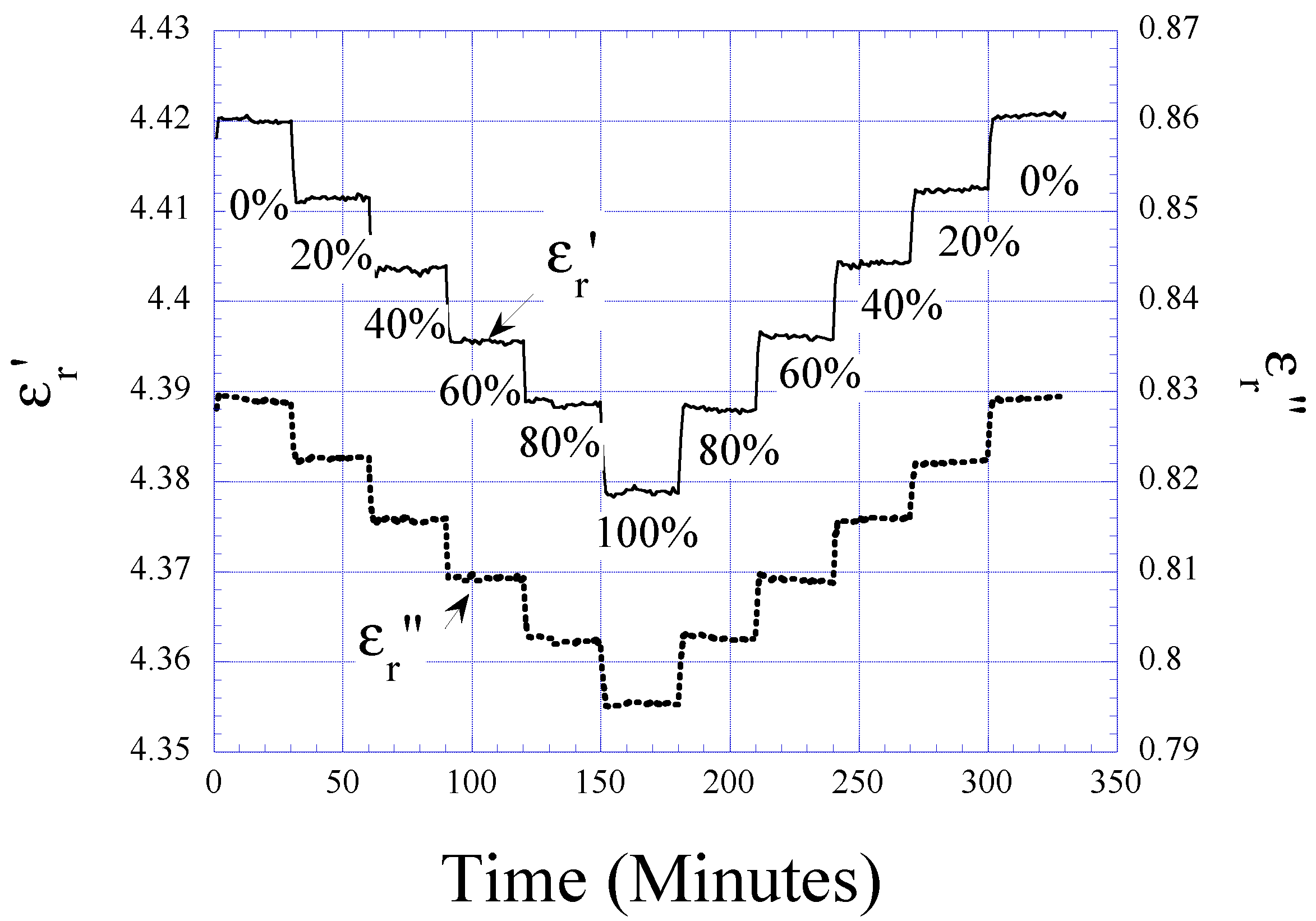
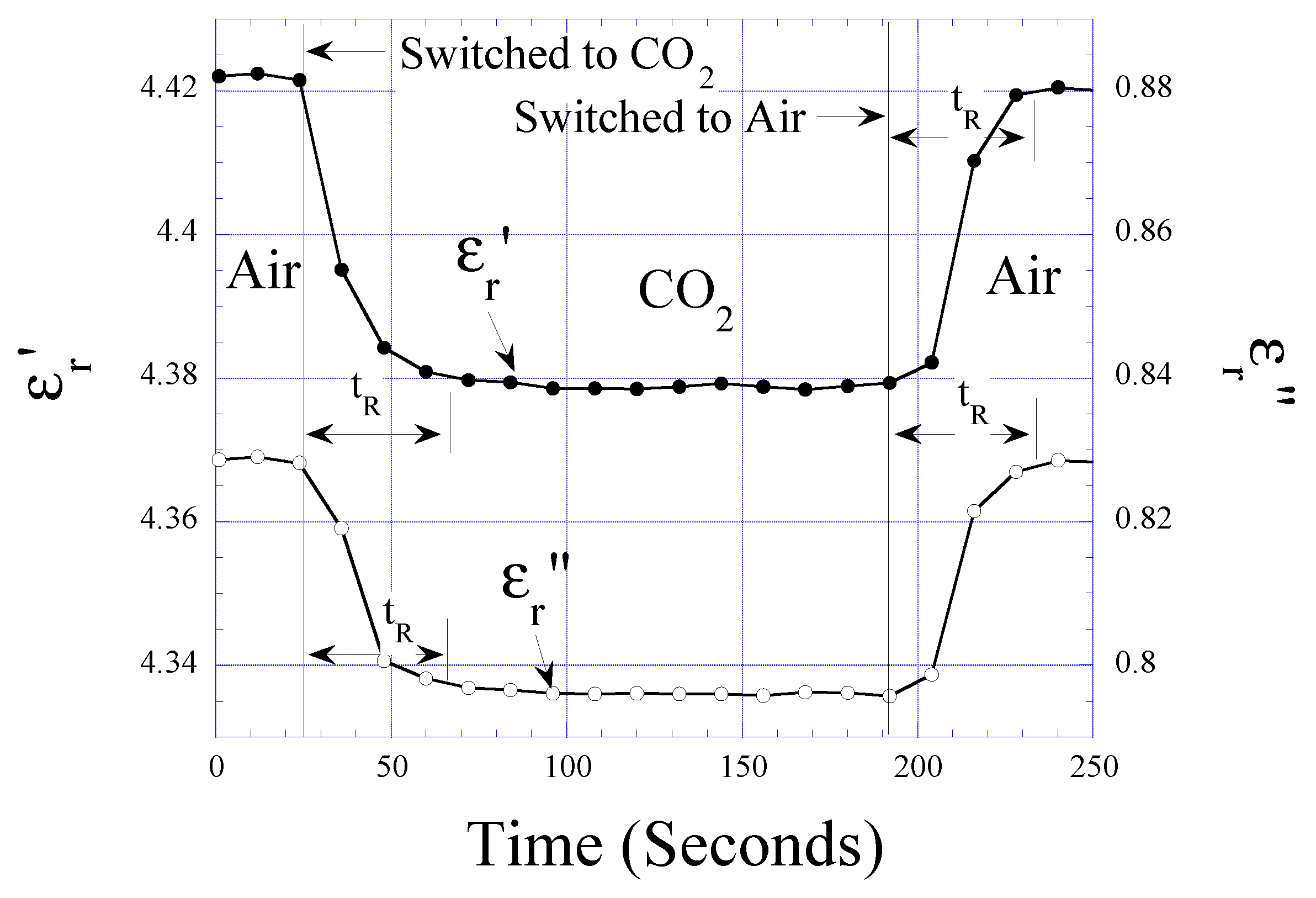
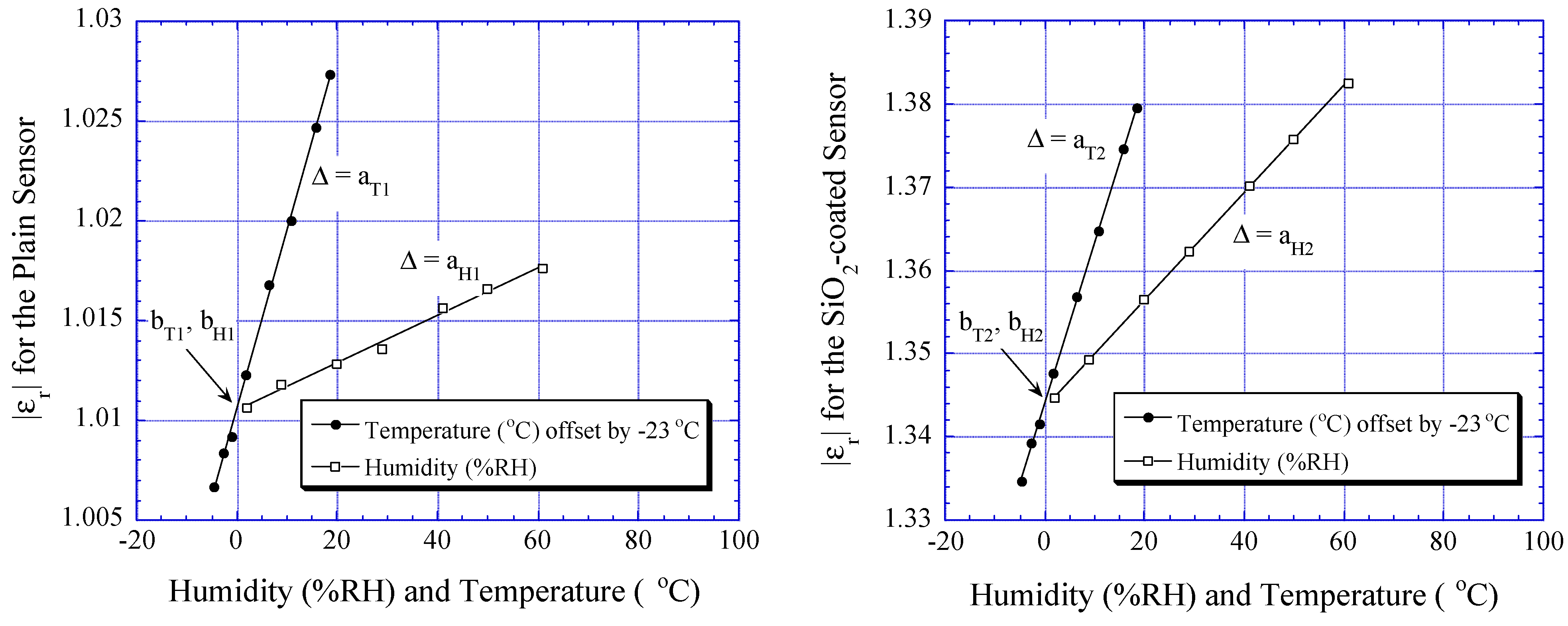
Humidity and Temperature Dependencies



| Coefficients | Plain Sensor | SiO2-coated Sensor | MWNT-coated Sensor |
|---|---|---|---|
| aT | aT1 = 0.00089523 | aT2 = 0.00193071 | aT3 = 0.024175 |
| bT | bT1 = 1.01062 | bT2 = 1.34392 | bT3 = 4.49524 |
| aH | aH1 = 0.00011883 | aH2 = 0.00064168 | aH3 = 0.0052665 |
| bH | bH1 = 1.01051 | bH2 = 1.34359 | bH3 = 4.49472 |
| aX | 0 | 0 | aX3 = -0.00045769 |
| bX | 0 | 0 | bX3 = 4.49562 |



Conclusions
Acknowledgements
References
- de Heer, W. A.; Chatelain, A.; and Ugarte, D. A carbon nanotube field-emission electron source. Science 1995, 270, 1179. [Google Scholar] [CrossRef]
- Tans, S.; Verschueren, A.; Dekker, C. Room-temperature transistor based on a single carbon nanotube. Nature 1998, 393, 49. [Google Scholar]
- Martel, R.; Schmidt, T.; Shea, H. R.; Hertel, T.; Avouris, P.h. Single- and multi-wall carbon nanotubes field-effect transistor. Appl. Phys. Lett. 1998, 73, 2447. [Google Scholar] [CrossRef]
- Tan, S.; Devoret, M.; Dai, H.; Thess, A.; Smalley, R.; Geerligs, L.; Dekker, C. Individual single- wall carbon nanotubes as quantum wires. Nature 1997, 386, 474. [Google Scholar]
- Yao, Z.; Postma, H.; Balents, L.; Dekker, C. Carbon nanotube intramolecular junctions. Nature 1999, 402, 273. [Google Scholar]
- Baughman, R. H.; Cui, C.; Zakhidov, M.; Iqbal, Z.; Barasci, J. N.; Spinks, G. M.; Wallace, G. G.; Mazzoldi, A.; Rossi, D.; Rinzler, A. G.; Jaschinski, O.; Roth, S.; Kertesz, M. Carbon Nanotube Actuators. Science 1999, 284, 1340–1342. [Google Scholar] [CrossRef]
- Rueckes, T.; Kim, K.; Joselevich, E.; Tseng, G. Y.; Cheung, C.; Lieber, C. M. Carbon Nanotube- Based Nonvolatile Random Access Memory for Molecular Computing. Science 2000, 289, 94–97. [Google Scholar] [CrossRef]
- Kong, J.; Franklin, N. R.; Zhou, C.; Chapline, M. G.; Peng, S.; Cho, K.; Dai, H. Nanotube Molecular Wires as Chemical Sensors. Science 2000, 287, 622–625. [Google Scholar] [CrossRef]
- Bienfait, M.; Asmussen, B.; Johnson, M.; Zeppenfeld, P. Methane mobility in carbon nanotubes. Surface Science 2000, 460, 243. [Google Scholar] [CrossRef]
- Ong, K. G.; Grimes, C. A. A Resonant Printed-circuit Sensor for Remote Query Monitoring of Environmental Parameters. Smart Mater. Struct. 2000, 9, 421–428. [Google Scholar] [CrossRef]
- Ong, K. G. Design And Application Of Planar Inductor-Capacitor Resonant Circuit Remote Query Sensors, University of Kentucky Dissertation, August 2000.
- Sheppard, N.; Tucker, R.; Salehi-Had, S. Design of a conductimetric pH microsensor based on reversibly swelling hydrogels. Sens. Actuators B 1993, 10, 73–77. [Google Scholar] [CrossRef]
- Dalgaard, P.; Mejlholm, O.; Huss, H. H. Application of an interactive approach for development of a microbial model predicting the shelf-life of packed fish. Int. J of Food Microbiology 1997, 38, 169–179. [Google Scholar] [CrossRef]
- Well, S. L.; DeSimone, J. CO2 Technology Platform: An Important Tool for Environmental Problem Solving. Angew. Chem. Int. Ed. 2001, 40, 518–527. [Google Scholar] [CrossRef]
- Lindgren, T.; Norback, D.; Anderson, K.; Dammstrom, B. Cabin environment and perception of cabin air quality among commercial aircrew. Aviation, Space, and Environmental Medicine 2000, 71(8), 774–482. [Google Scholar]
- Endres, H.-E.; Hartinger, R.; Schwaiger, M.; Gmelch, G.; Roth, M. A Capacitive CO2 Sensor System with Suppression of the Humidity Interference. Sens. Actuators B 1999, 57, 83–87. [Google Scholar] [CrossRef]
- Lee, M. S.; Meyer, J. U. A New Process for Fabricating CO2-Sensoing Layers Based on BaTiO3 and Additives. Sens. Actuators B 2000, 68, 293–299. [Google Scholar] [CrossRef]
- Matsubara, S.; Kaneko, S.; Morimoto, S.; Shimizu, S.; Ishihara, T.; Takita, Y. A Practical Capacitive Type CO2 Sensor Using CeO2/BaCO3/CuO Ceramics. Sens. Actuators B 2000, 65, 128–132. [Google Scholar] [CrossRef]
- Currie, J. F.; Essalik, A.; Marusic, J. C. Micromachined Thin Film Solid State Electrochemical CO2, NO2, and SO2 Gas Sensors. Sens. Actuators B 1999, 59, 235–241. [Google Scholar] [CrossRef]
- Chemat Technology, Inc. 9036 Winaetka Ave., Northridge, CA. http://www.chemat.com.
- Ramo, S.; Whinnery, J. R.; Van Duzer, T. Fields and Waves in Communication Electronics; John Wiley & Sons, Inc; Chap. 13; 1984. [Google Scholar]
- Brian, H. E. Printed Inductors and Capacitors. Tele-Tech & Electronic Industries 1954, 14, 68–69. [Google Scholar]
- Markx, G. H.; Davey, C. L. The Dielectric Properties of Biological Cells at Radiodfrequencies: Applications in Biotechnology. Enzyme and Microbial Technology 1999, 25, 161–171. [Google Scholar] [CrossRef]
- Endres, H.-E.; Drost, S. Optimization of the geometry of gas-sensitive interdigital capacitor. Sens. Actuators B 1991, 4, 95–98. [Google Scholar] [CrossRef]
- Andrews, R.; Jacques, D.; Rao, A. M.; Derbyshire, F.; Qian, Q.; Fan, F.; Dickey, E. C.; Chen, J. Continuous Production of Aligned Carbon Nanotubes: A step Closer to Commersial realization. Chem. Phys. Lett. 1999, 303, 467–474. [Google Scholar] [CrossRef]
- Marliere, C.; Poncharal, P.; Vaccarini, L.; Zahab, A. Effect of gas adsorption on the electrical properties of single-walled carbon nanotubes mats. Materials Research Soc. Sym. Proc. 2000, 539, 173–178. [Google Scholar]
- Walker, P. L., Jr. Chemistry and Physics of Carbon. Marcel Dekker, Inc.; vol. 6, New York, 1970. [Google Scholar]
- Sumanasekera, G. U.; Adu, C. K. W.; Fang, S.; Eklund, P. C. Effects of gas adsorption and collisions on electrical transport in single walled carbon nanotubes. Phy. Rev. Lett. 2000, 85, 1096–1099. [Google Scholar] [CrossRef] [PubMed]
- Kleinberg, J.; Argersinger, W. J., Jr; Griswold, E. Inorganic Chemistry; D.C. Health and Co.: Boston, 1960. [Google Scholar]
- Stepanek, I.; de Menorval, L. C.; Edwards, R.; Bernier, P. Carbon nanotubes and gas adsorption. AIP Conference Proc. 1999, 486, 456–461. [Google Scholar]
- Collins, P. G.; Bradley, P. G.; Ishigami, K.; Zettl, A. Extreme Oxygen Sensitivity of Electronic Properties of Carbon Nanotubes. Science 2000, 287, 1801–1804. [Google Scholar] [CrossRef]
- Sample Availability: Available from the author.
2001 by MDPI (http://www.mdpi.net). Reproduction is permitted for noncommercial purposes.
Share and Cite
Ong, K.G.; Grimes, C.A. A Carbon Nanotube-based Sensor for CO2 Monitoring. Sensors 2001, 1, 193-205. https://doi.org/10.3390/s10600193
Ong KG, Grimes CA. A Carbon Nanotube-based Sensor for CO2 Monitoring. Sensors. 2001; 1(6):193-205. https://doi.org/10.3390/s10600193
Chicago/Turabian StyleOng, Keat G., and Craig A. Grimes. 2001. "A Carbon Nanotube-based Sensor for CO2 Monitoring" Sensors 1, no. 6: 193-205. https://doi.org/10.3390/s10600193
APA StyleOng, K. G., & Grimes, C. A. (2001). A Carbon Nanotube-based Sensor for CO2 Monitoring. Sensors, 1(6), 193-205. https://doi.org/10.3390/s10600193




