Discrimination of Apple Liqueurs (Nalewka) Using a Voltammetric Electronic Tongue, UV-Vis and Raman Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
2.2. Apple Nalewka Samples
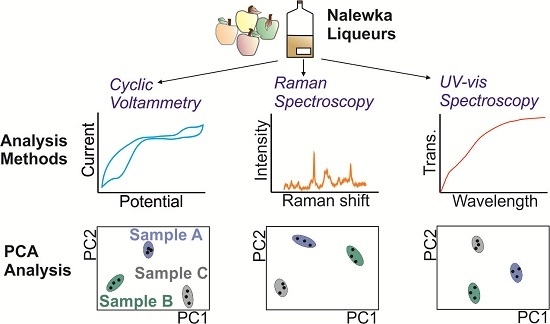
2.3. Electronic Tongue, Raman Spectroscopy and UV-Vis Analysis
2.4. Data Preprocessing and Statistical Analysis
3. Results and Discussion
3.1. Analysis of Density and Phenolic Content
3.2. Analysis of Liqueurs Using an E-Tongue
3.3. Analysis of Liqueurs Using UV-Vis and Color Analysis
3.4. Analysis of Liqueurs Using Raman Spectroscopy
3.5. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Śliwińska, M.; Wiśniewska, P.; Dymerski, T.; Wardencki, W.; Namieśnik, J. The flavour of fruit spirits and fruit liqueurs: A review. Flavour Frag. J. 2015, 30, 197–207. [Google Scholar] [CrossRef]
- Wiśniewska, P.; Śliwińska, M.; Dymerski, T.; Wardencki, W.; Namieśnik, J. Application of gas chromatography to analysis of spirit-based alcoholic beverages. Crit. Rev. Anal. Chem. 2015, 45, 201–225. [Google Scholar] [CrossRef] [PubMed]
- Polak, J.; Bartoszek, M. The study of antioxidant capacity of varieties of nalewka, a traditional Polish fruit liqueur, using EPR, NMR and UV-vis spectroscopy. J. Food Comp. Anal. 2015, 40, 114–119. [Google Scholar] [CrossRef]
- Beebe, K.R.; Pell, R.J.; Seasholtz, M.B. Chemometrics: A Practical Guide; Wiley-Interscience Series: New York, NY, USA, 1998. [Google Scholar]
- Escarpa, A.; Gonzalez, M.G.; Lopez, M.A. Agricultural and Food Electroanalysis; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Wu, Z.; Long, J.; Xu, E.; Wang, F.; Xu, X.; Jin, Z.; Jiao, A. Rapid measurement of antioxidant activity and γ-aminobutyric acid content of Chinese rice wine by Fourier-Transform Near Infrared Spectroscopy. Food Anal. Methods 2015, 8, 2541–2553. [Google Scholar] [CrossRef]
- Toko, K.; Tahara, Y.; Habara, M.; Kobayashi, Y.; Hikezaki, H. Taste Sensor: Electronic Tongue with Global Selectivity in Essentials of Machine Olfaction and Taste; Wiley: Hoboken, NJ, USA, 2016. [Google Scholar]
- Rodríguez-Méndez, M.L.; Medina-Plaza, C.; García-Hernández, C.; Rodriguez, S.; García-Cabezón, C.; Paniagua, D.; Rodríguez-Pérez, M.A.; de Saja, J.A. Improvement of electrocatalytic effect in voltammetric sensors based on phthalocyanines. J. Porphyr. Phthalocyanines 2016, 20, 1–6. [Google Scholar] [CrossRef]
- Rodriguez-Mendez, M.L. Electronic Noses and Tongues in Food Science; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Cetó, X.; Llobet, M.; Marco, J.; Del Valle, M. Application of an electronic tongue towards the analysis of brandies. Anal. Methods 2013, 5, 1120–1129. [Google Scholar] [CrossRef]
- Arrieta, A.A.; Rodríguez-Méndez, M.L.; De Saja, J.A.; Blanco, C.A.; Nimubona, D. Prediction of bitterness and alcoholic degree in beer using an electronic tongue. Food Chem. 2010, 123, 642–646. [Google Scholar] [CrossRef]
- Tahara, Y.; Nakashi, K.; Ji, K.; Ikeda, A.; Toko, K. Development of a portable taste sensor with a lipid/polymer membrane. Sensors 2013, 13, 1076–1087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raithore, S.; Bai, J.; Plotto, A.; Manthey, J.; Irey, M.; Baldwin, E. Electronic tongue response to chemicals in orange juice that change concentration in relation to harvest maturity and citrus greening or Huanglongbing (HLB) Disease. Sensors 2015, 15, 30062–30075. [Google Scholar] [CrossRef] [PubMed]
- Artigas, J.; Jimenez, C.; Dominguez, C.; Minguez, S.; Gonzalo, A.; Alonso, J. Development of a multiparametric analyser based on ISFET sensors applied to process control in the wine industry. Sens. Actuators B Chem. 2003, 89, 199–204. [Google Scholar] [CrossRef]
- Gutiérrez, M.; Llobera, A.; Vila-Planas, J.; Capdevila, F.; Demming, S.; Büttgenbach, S.; Mínguez, S.; Jiménez-Jorquera, C. Hybrid electronic tongue based on optical and electrochemical microsensors for quality control of wine. Analyst 2010, 135, 1718–1725. [Google Scholar] [CrossRef] [PubMed]
- Kirsanov, D.; Mednova, O.; Vietoris, V.; Kilmartin, P.A.; Legin, A. Towards reliable estimation of an “electronic tongue” predictive ability from PLS regression models in wine analysis. Talanta 2012, 90, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mendez, M.L.; Apetrei, C.; Gay, M.; Medina-Plaza, C.; De Saja, J.A.; Vidal, S.; Aagaard, O.; Wirth, J.; Cheynier, V. Evaluation of oxygen exposure levels and polyphenolic content of red wines using an electronic panel formed by an electronic nose and an electronic tongue. Food Chem. 2014, 155, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Blanco, C.; De la Fuente, R.; Caballero, I.; Rodríguez-Méndez, M.L. Beer discrimination using a portable electronic tongue based on screen-printed electrodes. J. Food Eng. 2015, 157, 57–62. [Google Scholar] [CrossRef]
- Górski, Ł.; Sordoń, W.; Ciepiela, F.; Kubiak, W.W.; Jakubowska, M. Voltammetric classification of ciders with PLS-DA. Talanta 2015, 46, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Legin, A.; Rudnitskaya, A.; Seleznev, B.; Vlasov, Y. Electronic tongue for quality assessment of ethanol, vodka and eau-de-vie. Anal. Chim. Acta 2005, 534, 129–135. [Google Scholar] [CrossRef]
- Lvova, L.; Paolesse, R.; Di Natale, C.; D’Amico, A. Detection of alcohols in beverages: An application of porphyrin-based Electronic tongue. Sens. Actuators B Chem. 2006, 118, 439–447. [Google Scholar] [CrossRef]
- Śliwińska, M.; Wiśniewska, P.; Dymerski, T.; Wardencki, W.; Namieśnik, J. Application of electronic nose based on fast GC for authenticity assessment of Polish homemade liqueurs called nalewka. Food Anal. Methods 2016. [Google Scholar] [CrossRef]
- Bokszczanin, K.L.; Przybyła, A.A.; Kreszdorn, N.; Winter, P.; Filipecki, M. Investigation of Mal d 1 Allelic variants and phylogenetic diversity in contemporary and historical Polish apple cultivars. J. Agric. Sci. 2015, 7, 115–131. [Google Scholar] [CrossRef]
- Dobrowolska-Iwanek, J.; Gąstoł, M.; Adamska, A.; Krośniak, M.; Zagrodzki, P. Traditional versus modern apple cultivars—A comparison of juice composition. Folia Hort. 2015, 27, 33–41. [Google Scholar] [CrossRef]
- Apetrei, C.; Apetrei, I.M.; De Saja, J.A.; Rodriguez-Méndez, M.L. Carbon paste electrodes made from different carbonaceous materials: application in the study of antioxidants. Sensors 2011, 11, 1328–1344. [Google Scholar]
- Medina-Plaza, C.; García-Hernandez, C.; De Saja, J.A.; Fernandez-Escudero, J.A.; Barajas, E.; Medrano, G.; García-Cabezon, C.; Martin-Pedrosa, F.; Rodriguez-Mendez, M.L. The advantages of disposable screen-printed biosensors in a bioelectronic tongue for the analysis of grapes. LWT Food Sci. Technol. 2015, 62, 940–947. [Google Scholar] [CrossRef]
- Central Bureau of the CIE. Colorimetry, 2nd ed.; CIE Publication: Vienna, Austria, 1986. [Google Scholar]
- Stratil, P.; Kuban, V.; Fojtova, J. Comparison of the phenolic content and total antioxidant activity in wines as determined by spectrophotometric methods. Czech J. Food Sci. 2008, 26, 242–253. [Google Scholar]
- Apetrei, I.M.; Rodríguez-Méndez, M.L.; Apetrei, C.; Nevares, I.; Del Alamo, M.; De Saja, J.A. Monitoring of evolution during red wine aging in oak barrels and alternative method by means of an electronic panel test. Food Res. Int. 2012, 45, 244–249. [Google Scholar] [CrossRef]
- Medina-Plaza, C.; Garcia-Cabezon, C.; Garcia-Hernandez, C.; Bramorski, C.; Blanco-Val, Y.; Martin-Pedrosa, F.; Kawai, T.; De Saja, J.A.; Rodriguez-Mendez, M.L. Analysis of organic acids and phenols of interest in the wine industry using Langmuir-Blodgett films based on functionalized nanoparticles. Anal. Chim. Acta 2015, 853, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Gallego, A.L.; Guesalaga, A.R.; Bordeu, E.; Gonzalez, A.S. Rapid measurement of phenolics compounds in red wine using Raman spectroscopy. IEEE Trans. Instrum. Meas. 2011, 60, 507–512. [Google Scholar] [CrossRef]
- Fernández-González, A.; Montejo-Bernardo, J.M.; Rodríguez-Prieto, H.; Castaño-Monllor, C.; Badía-Laíño, R.; Díaz-García, M.E. Easy-to-use analytical approach based on ATR-FTIR and chemometrics to identify apple varieties under Protected Designation of Origin (PDO). Comput. Electron. Agric. 2014, 108, 166–172. [Google Scholar] [CrossRef]
- Yang, D.; Ying, Y. Applications of Raman spectroscopy in agricultural products and food analysis: A review. Appl. Spectrosc. Rev. 2011, 46, 539–560. [Google Scholar] [CrossRef]
- Boyaci, I.H.; Genis, H.E.; Guven, B.; Tamer, U.; Alper, N. A novel method for quantification of ethanol and methanol in distilled alcoholic beverages using Raman spectroscopy. J. Raman Spectrosc. 2012, 43, 1171–1176. [Google Scholar] [CrossRef]
- Martina, C.; Bruneel, J.L.; Guyon, F.; Médina, B.; Jourdes, M.; Teissedre, P.L.; Guillaume, F. Raman spectroscopy of white wines. Food Chem. 2015, 181, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Winquist, F. Voltammetric electronic tongues—Basic principles and applications. Microchim. Acta 2008, 163, 3–10. [Google Scholar] [CrossRef]
- Prieto, N.; Oliveri, P.; Leardi, R.; Gay, M.; Apetrei, C.; Rodriguez-Méndez, M.L.; De Saja, J.A. Application of a GA–PLS strategy for variable reduction of electronic tongue signals. Sens. Actuators B Chem. 2013, 183, 52–57. [Google Scholar] [CrossRef]
- Cetó, X.; Apetrei, C.; del Valle, M.; Rodriguez-Mendez, M.L. Evaluation of red wines antioxidant capacity by means of a voltammetric e-tongue with an optimized sensor array. Electrochim. Acta 2012, 120, 180–186. [Google Scholar] [CrossRef]
- Hori, R.; Sugiyama, J. A combined FT-IR microscopy and principal component analysis on softwood cell walls. Carbohydr. Polym. 2003, 52, 449–453. [Google Scholar] [CrossRef]
- Śliwińska, M.; Wiśniewska, P.; Dymerski, T.; Wardencki, W.; Namieśnik, J. Evaluation of the suitability of electronic nose based on fast GC for distinguishing between the plum spirits of different geographical origins. Eur. Food Res. Technol. 2016. [Google Scholar] [CrossRef]






| Apple Variety | Phenolic Content (mg·gallic·acid/L) | Density (g/cm3) |
|---|---|---|
| Ligol | 661.01 | 1.1061 |
| Kosztela | 767.81 | 1.0982 |
| Grey Reinette | 1005.98 | 1.0955 |
| Rubin | 783.88 | 1.0946 |
| Cox Orange | 600.52 | 1.0995 |
| Apple Variety | L* | a* | b* | C* | h* [°] | BI |
|---|---|---|---|---|---|---|
| Ligol | 87.74 ± 0.01 | −0.10 ± 0.01 | 31.36 ± 0.01 | 31.36 ± 0.01 | 90.18 ± 0.01 | 42.39 ± 0.00 |
| Kosztela | 87.52 ± 0.01 | 1.16 ± 0.01 | 32.45 ± 0.01 | 32.47 ± 0.01 | 87.96 ± 0.01 | 45.49 ± 0.01 |
| Grey Reinette | 87.05 ± 0.01 | 0.41 ± 0.01 | 48.50 ± 0.01 | 48.50 ± 0.01 | 89.52 ± 0.01 | 76.64 ± 0.02 |
| Rubin | 92.52 ± 0.01 | −0.34 ± 0.01 | 28.02 ± 0.01 | 28.02 ± 0.01 | 90.69 ± 0.01 | 34.46 ± 0.01 |
| Cox Orange | 92.91 ± 0.01 | −1.43 ± 0.01 | 22.00 ± 0.01 | 22.04 ± 0.01 | 93.71 ± 0.01 | 24.90 ± 0.00 |
| Relation between Groups | E-Tongue | CIELab Parameters | Raman Spectra | ||||
|---|---|---|---|---|---|---|---|
| E. Distance | CV | E. Distance | CV | E. Distance | CV | ||
| Ligol | Kosztela | 5899.50 | 0.06 | 4.19 | <0.01 | 28.83 | 0.15 |
| Ligol | Grey Reinette | 2808.12 | 0.15 | 41.97 | <0.01 | 34.86 | 0.18 |
| Ligol | Cox Orange | 6064.19 | 0.06 | 22.81 | <0.01 | 21.88 | 0.29 |
| Ligol | Rubin | 5066.53 | 0.10 | 10.60 | <0.01 | 20.34 | 0.23 |
| Kosztela | Grey Reinette | 6514.71 | 0.05 | 38.57 | <0.01 | 20.07 | 0.12 |
| Kosztela | Rubin | 3238.44 | 0.14 | 13.97 | <0.01 | 8.94 | 0.07 |
| Kosztela | Cox Orange | 10191.66 | 0.04 | 26.66 | <0.01 | 8.58 | 0.36 |
| Grey Reinette | Rubin | 5916.40 | 0.09 | 51.48 | <0.01 | 19.26 | 0.13 |
| Grey Reinette | Cox Orange | 5115.47 | 0.07 | 64.30 | <0.01 | 17.25 | 0.24 |
| Rubin | Cox Orange | 8118.85 | 0.06 | 13.17 | <0.01 | 2.13 | 1.17 |
| Voltammetric Outputs | |||||
| Parameters | R2C (a) | RMSEC (b) | R2P (c) | RMSEP (d) | Latent Variables |
| Polyphenolic content (Folin–Ciocalteu method) | 0.976744 | 29.75508 | 0.939679 | 47.74666 | 4 |
| Density | 0.925237 | 0.001112 | 0.878397 | 0.001751 | 4 |
| CIELab Outputs | |||||
| Parameters | R2C (a) | RMSEC (b) | R2P (c) | RMSEP (d) | Latent Variables |
| Polyphenolic content (Folin–Ciocalteu method) | 0.996525 | 8.180929 | 0.994252 | 11.27284 | 3 |
| Density | 0.879691 | 0.001410 | 0.807301 | 0.001912 | 3 |
| Raman Outputs | |||||
| Parameters | R2C (a) | RMSEC (b) | R2P (c) | RMSEP (d) | Latent Variables |
| Polyphenolic content (Folin–Ciocalteu method) | 0.906231 | 42.49629 | 0.793365 | 67.59048 | 6 |
| Density | 0.962399 | 0.000788 | 0.856644 | 0.001650 | 3 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Śliwińska, M.; Garcia-Hernandez, C.; Kościński, M.; Dymerski, T.; Wardencki, W.; Namieśnik, J.; Śliwińska-Bartkowiak, M.; Jurga, S.; Garcia-Cabezon, C.; Rodriguez-Mendez, M.L. Discrimination of Apple Liqueurs (Nalewka) Using a Voltammetric Electronic Tongue, UV-Vis and Raman Spectroscopy. Sensors 2016, 16, 1654. https://doi.org/10.3390/s16101654
Śliwińska M, Garcia-Hernandez C, Kościński M, Dymerski T, Wardencki W, Namieśnik J, Śliwińska-Bartkowiak M, Jurga S, Garcia-Cabezon C, Rodriguez-Mendez ML. Discrimination of Apple Liqueurs (Nalewka) Using a Voltammetric Electronic Tongue, UV-Vis and Raman Spectroscopy. Sensors. 2016; 16(10):1654. https://doi.org/10.3390/s16101654
Chicago/Turabian StyleŚliwińska, Magdalena, Celia Garcia-Hernandez, Mikołaj Kościński, Tomasz Dymerski, Waldemar Wardencki, Jacek Namieśnik, Małgorzata Śliwińska-Bartkowiak, Stefan Jurga, Cristina Garcia-Cabezon, and Maria Luz Rodriguez-Mendez. 2016. "Discrimination of Apple Liqueurs (Nalewka) Using a Voltammetric Electronic Tongue, UV-Vis and Raman Spectroscopy" Sensors 16, no. 10: 1654. https://doi.org/10.3390/s16101654