Disposable, Paper-Based, Inkjet-Printed Humidity and H2S Gas Sensor for Passive Sensing Applications
Abstract
:1. Introduction
2. Sensor Design and Working Principle
2.1. Paper Substrate
2.2. Interdigitated Electrode
2.3. Chemi-Resistive Ink
3. Antenna Design
4. Experimental Results
- -
- A gas line with a flow control valve coming from the H2S gas cylinder with controllable concentration. The Mass Flow Controller (MFC) is provided by Alicat Scientific Inc. (Tucson, AZ, USA).
- -
- An aluminum chamber for housing the sensor with two leads for external connections. In addition, the chamber consists of commercial humidity sensor (Honeywell HIH-4000-003) for calibration purposes.
- -
- The chamber is connected to an LCR meter (Agilent E4980A) that is accessed by a PC through RS-232 to USB interface and National Instruments (NI) LabVIEW software (ver. 2011).
4.1. Antenna Characterization
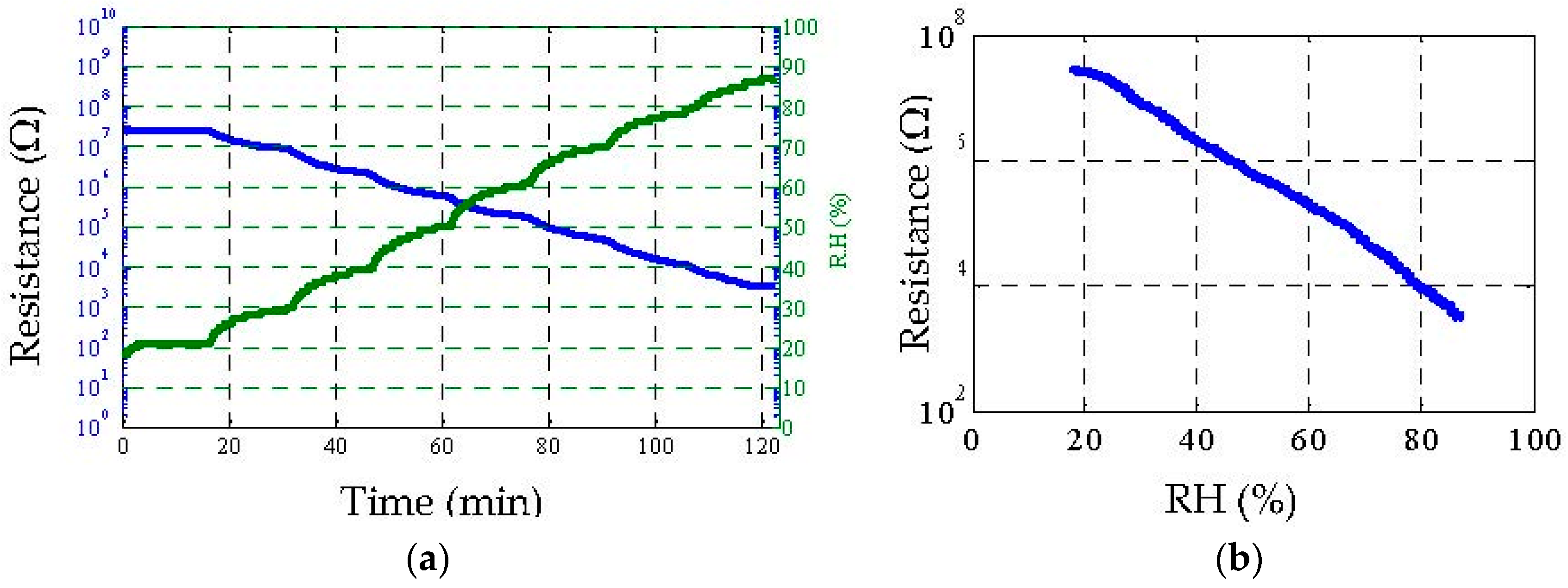
4.2. Humidity Sensing
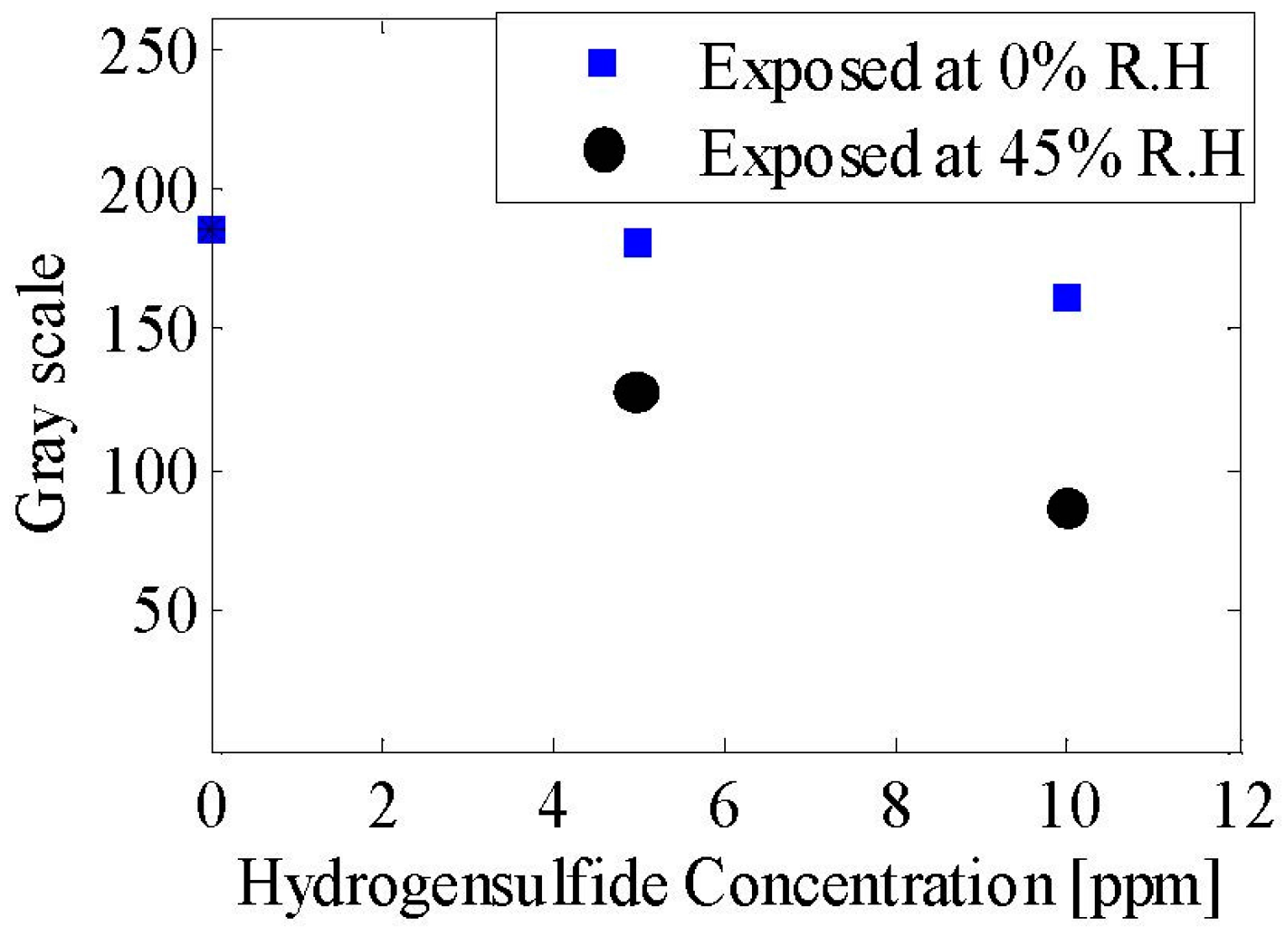
4.3. Gas Sensing
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- A Guide to Internet of Things; Intel Corporation: Santa Clara, CA, USA, 2010.
- Amendola, S.; Lodato, R.; Manzari, S.; Occhiuzzi, C.; Marrocco, G. RFID Technology for IoT-Based Personal Healthcare in Smart Spaces. IEEE Internet Things J. 2014, 1, 144–152. [Google Scholar] [CrossRef] [Green Version]
- Pasluosta, C.F.; Gassner, H.; Winkler, J.; Klucken, J.; Eskofier, B.M. An Emerging Era in the Management of Parkinson’s Disease: Wearable Technologies and the Internet of Things. IEEE J. Biomed. Health Inform. 2015, 16, 1873–1881. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Yan, G.; Xu, L.D. Developing Vehicular Data Cloud Services in the IoT Environment. IEEE Trans. Ind. Inform. 2014, 10, 1587–1595. [Google Scholar] [CrossRef]
- Chan, M.; Estève, D.; Escriba, C.; Campo, E. A review of smart homes—Present state and future challenges. Comput. Methods Prog. Biomed. 2008, 91, 55–81. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.; Campo, E.; Estève, D.; Fourniols, J.Y. Smart homes—Current features and future perspectives. Maturitas 2009, 64, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.D.T.; Suryadevara, N.K.; Mukhopadhyay, S.C. Towards the Implementation of IoT for Environmental Condition Monitoring in Homes. IEEE Sens. J. 2013, 13, 3846–3853. [Google Scholar] [CrossRef]
- Zanella, A.; Bui, N.; Castellani, A.; Vangelista, L.; Zorzi, M. Internet of Things for Smart Cities. IEEE Internet Things J. 2014, 1, 22–32. [Google Scholar] [CrossRef]
- Perera, C.; Liu, C.H.; Jayawardena, S.; Chen, M. A Survey on Internet of Things from Industrial Market Perspective. IEEE Access 2014, 2, 1660–1679. [Google Scholar] [CrossRef]
- Andersson, H.; Manuilskiy, A.; Gao, J.; Lidenmark, C.; Siden, J.; Forsberg, S.; Unander, T.; Nilsson, H.-E. Investigation of humidity sensor effect in silver nanoparticle ink sensors printed on paper. IEEE Sens. J. 2013, 14, 623–628. [Google Scholar] [CrossRef]
- Gao, J.; Sidén, J.; Nilsson, H.-E.; Gulliksson, M. Printed Humidity Sensor with Memory Functionality for Passive RFID Tags. IEEE Sens. J. 2013, 13, 1824–1834. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, R.; Staiculescu, D.; Wong, C.; Tentzeris, M. A novel conformal RFID-enabled module utilizing inkjet-printed antennas and carbon nanotubes for gas-detection applications. IEEE Antennas Wirel. Propag. Lett. 2009, 8, 653–656. [Google Scholar] [CrossRef]
- Sarfraz, J.; Maattanen, A.; Ihalainen, P.; Keppeler, M.; Lindén, M.; Peltonen, J. Printedcopper acetate based H2S sensor on paper substrate. Sens. Actuators B Chem. 2012, 173, 868–873. [Google Scholar] [CrossRef]
- Harrey, P.; Ramsey, B.; Evans, P.; Harrison, D. Capacitive-type humidity sensors fabricated using the offset lithographic printing process. Sens. Actuators B Chem. 2002, 87, 226–232. [Google Scholar] [CrossRef]
- Molina-Lopez, F.; Briand, D.; de Rooij, N. All additive inkjet printed humidity sensors on plastic substrate. Sens. Actuators B Chem. 2012, 166, 212–222. [Google Scholar] [CrossRef]
- Rivadeneyra, A.; Fernández-Salmerón, J.; Banqueri, J.; López-Villanueva, J.A.; Capitan-Vallvey, L.F.; Palma, A.J. A novel electrode structure compared with interdigitated electrodes as capacitive sensor. Sens. Actuators B Chem. 2014, 204, 552–560. [Google Scholar] [CrossRef]
- Weremczuk, J.; Tarapata, G.; Jachowicz, R.S. The ink-jet printing humidity sorption sensor—Modelling, design, technology and characterization. Meas. Sci. Technol. 2012, 23, 014003. [Google Scholar] [CrossRef]
- Molina-Lopez, F.; Quintero, A.V.; Mattana, G.; Briand, D.; de Rooij, N. Large-area compatible fabrication and encapsulation of inkjet-printed humidity sensors on flexible foils with integrated thermal compensation. J. Micromech. Microeng. 2013, 23, 025012. [Google Scholar] [CrossRef]
- Rivadeneyra, A.; Fernández-Salmerón, J.; Agudo-Acemel, M.; López-Villanueva, J.A.; Capitan-Vallvey, L.F.; Palma, A.J. Printed electrodes structures as capacitive humidity sensors: A comparison. Sens. Actuators A Phys. 2016, 244, 56–65. [Google Scholar] [CrossRef]
- Doujaiji, B.; Al-Tawfiq, J.A. Hydrogen sulfide exposure in an adult male. Ann. Saudi Med. 2010, 30, 76–80. [Google Scholar] [PubMed]
- Khan, M.M.; Tahir, F.A.; Farooqui, M.F.; Shamim, A.; Cheema, H.M. 3.56 bits/cm2 Compact Inkjet Printed and Application Specific Chipless RFID Tag. IEEE Antenna Wirel. Propag. Lett. 2016, 15, 1109–1112. [Google Scholar] [CrossRef]
- Vena, A.; Perret, E.; Tedjini, S.; Tourtollet, G.M.P.; Delattre, A.; Garet, F.; Boutant, Y. Design of Chipless RFID Tags Printed on Paper by Flexography. IEEE Trans. Antennas Propag. 2013, 61, 5868–5877. [Google Scholar] [CrossRef]
- Sarfraz, J.; Tobjork, D.; Osterbacka, R.; Linden, M. Low-cost hydrogen sulfide gas sensor on paper substrates: Fabrication and demonstration. IEEE Sens. J. 2012, 12, 1973–1978. [Google Scholar] [CrossRef]
- Ahmed, S.; Tahir, F.A.; Shamim, A.; Cheema, H.M. A Compact Kapton-Based Inkjet Printed Multiband Antenna for Flexible Wireless Devices. IEEE Antenna Wirel. Propag. Lett. 2015, 14, 1802–1805. [Google Scholar] [CrossRef]
- Sarfraza, J.; Ihalainena, P.; Maattanena, A.; Gulina, T.; Koskelab, J.; Wilénc, C.E.; Kilpeläb, A.; Peltonenaa, J. A printed H2S sensor with electro-optical response. Sens. Actuators B Chem. 2014, 191, 821–827. [Google Scholar] [CrossRef]
- Tobjörk, D.; Österbacka, R. Paper Electronics. Adv. Mater. 2011, 23, 1935–1961. [Google Scholar] [CrossRef] [PubMed]
- Virji, S.; Kaner, R.; Weiller, B. Direct electrical measurement of the conversion of metal acetates to metal sulfides by hydrogen sulfide. Inorg. Chem. 2006, 45, 10467–10471. [Google Scholar] [CrossRef] [PubMed]
- Sapsanis, C.; Omran, H.; Chernikova, V.; Shekhah, O.; Belmabkhout, Y.; Buttner, U.; Eddaoudi, M.; Salama, K.N. Insights on Capacitive Interdigitated Electrodes Coated with MOF Thin Films: Humidity and VOCs Sensing as a Case Study. Sensors 2015, 15, 18153–18166. [Google Scholar] [CrossRef] [PubMed]
- Omran, H.; Arsalan, M.; Salama, K.N. An Integrated Energy-Efficient Capacitive Sensor Digital Interface Circuit. Sens. Actuators A Phys. 2014, 216, 43–51. [Google Scholar] [CrossRef]
- Abeles, B.; Pinch, H.L.; Gittleman, J.I. Percolation conductivity in W-Al2O3 granular metal films. Phys. Rev. Lett. 1975, 35, 247–250. [Google Scholar] [CrossRef]
- Sheng, P.; Abeles, B.; Arie, Y. Hopping conductivity in granular metals. Phys. Rev. Lett. 1973, 31, 44–47. [Google Scholar] [CrossRef]












| Parameter | Dimension | Parameter | Dimension |
|---|---|---|---|
| L0 | 50.25 mm | W0 | 35 mm |
| L1 | 1.64 mm | W1 | 10.75 mm |
| L2 | 13.75 mm | W2 | 3.00 mm |
| L3 | 14.57 mm | W3 | 2.75 mm |
| L4 | 3.5 mm | W4 | 14 mm |
| L5 | 45.75 mm | W5 | 20 mm |
| L6 | 20.5 mm | L7 | 20 mm |
| L6 | L7 | |
|---|---|---|
| Tag 1 | 20.5 mm | 20 mm |
| Tag 2 | 21.5 mm | 21 mm |
| Tag 3 | 22.5 mm | 22 mm |
| Tag 4 | 23.5 mm | 23 mm |
| Tag 5 | 24.5 mm | 24 mm |
| Reference | Sensor Type | Tag Size | Response Time | Substrate | Integrated with Antenna | Identification Capability |
|---|---|---|---|---|---|---|
| [10] | Humidity, Resistive type | 2.9 mm × 0.6 mm | More than 60 min | Paper | No | No |
| [11] | Humidity, Resistive type | 188 mm × 9 mm | 20–100 min | Paper | Yes | No |
| [12] | Gas (Ammonia & Nitrogen Oxide), Resistive type | 118 mm × 27 mm | - | Paper | Yes | No |
| [13] | Gas (Hydrogen Sulfide), Resistive type | 20 mm × 11 mm | Approx. 20 min | Paper | No | No |
| [14] | Humidity, Capacitive type | Sensor area is 200 mm2 | 4–11 min | Polyimide and Polyethersulphone | No | No |
| [15] | Humidity, Capacitive type | 12 mm × 8 mm | Approx. 24 s | PET | No | No |
| [16] | Humidity, Capacitive type | 6.3 mm × 1.85 mm | 5–6 min | Polyimide | No | No |
| [17] | Humidity, With both resistive and capacitive measurements | 5.4 mm × 5.4 mm | Less than 20 s | Polyimide | No | No |
| [18] | Humidity, Capacitive type | 12 mm × 12 mm | Approx. 20 min | PET | No | No |
| This work | Dual functionality Humidity & Gas (Hydrogen Sulfide), Resistive type | 45.75 mm × 20 mm | 3 min | Paper | Yes | Yes |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quddious, A.; Yang, S.; Khan, M.M.; Tahir, F.A.; Shamim, A.; Salama, K.N.; Cheema, H.M. Disposable, Paper-Based, Inkjet-Printed Humidity and H2S Gas Sensor for Passive Sensing Applications. Sensors 2016, 16, 2073. https://doi.org/10.3390/s16122073
Quddious A, Yang S, Khan MM, Tahir FA, Shamim A, Salama KN, Cheema HM. Disposable, Paper-Based, Inkjet-Printed Humidity and H2S Gas Sensor for Passive Sensing Applications. Sensors. 2016; 16(12):2073. https://doi.org/10.3390/s16122073
Chicago/Turabian StyleQuddious, Abdul, Shuai Yang, Munawar M. Khan, Farooq A. Tahir, Atif Shamim, Khaled N. Salama, and Hammad M. Cheema. 2016. "Disposable, Paper-Based, Inkjet-Printed Humidity and H2S Gas Sensor for Passive Sensing Applications" Sensors 16, no. 12: 2073. https://doi.org/10.3390/s16122073






