Radio-Frequency-Controlled Urea Dosing for NH3-SCR Catalysts: NH3 Storage Influence to Catalyst Performance under Transient Conditions
Abstract
:1. Introduction
2. Radio-Frequency-Based (RF) Catalyst State Monitoring
3. Experimental Section
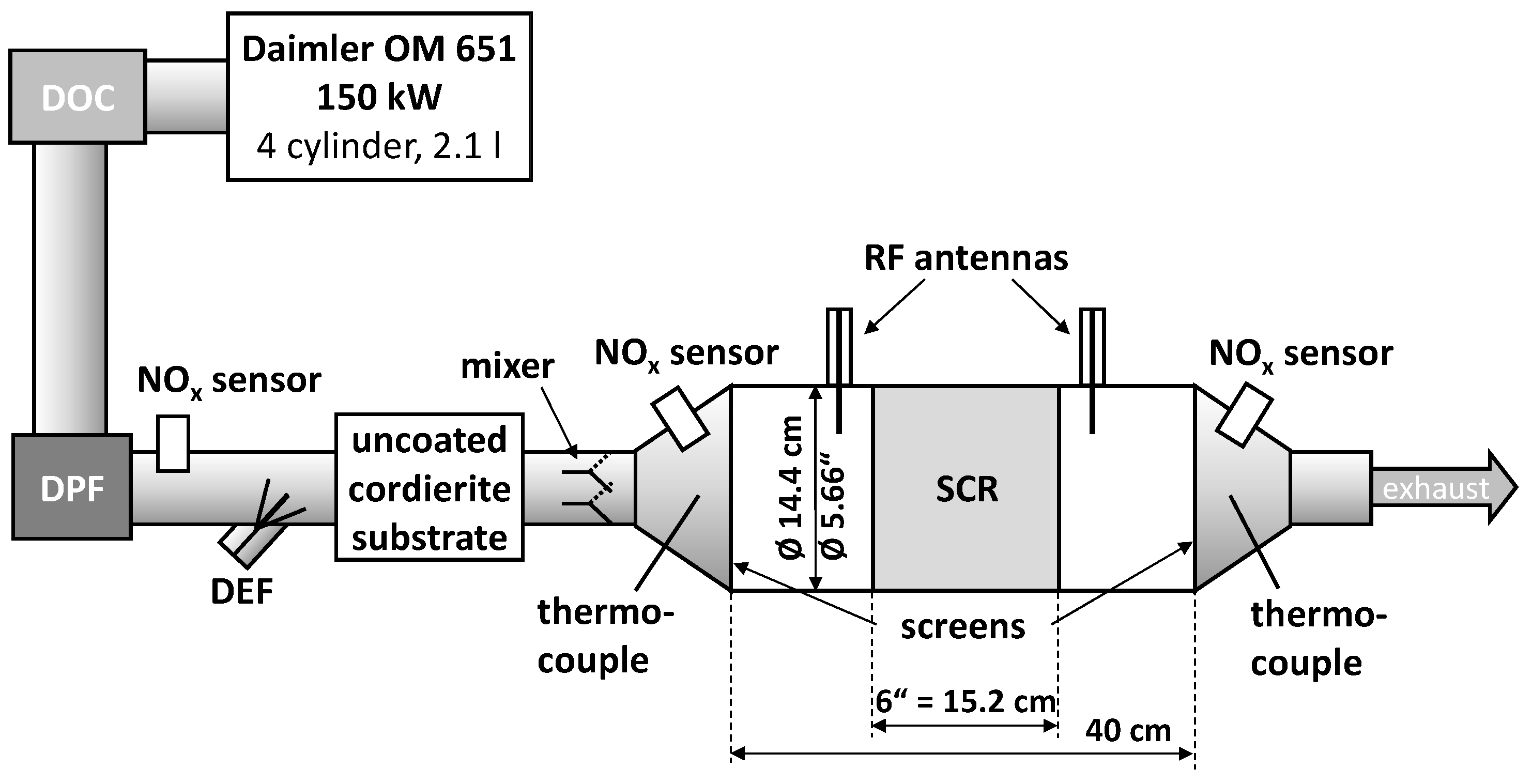
3.1. Dynamometer Setup
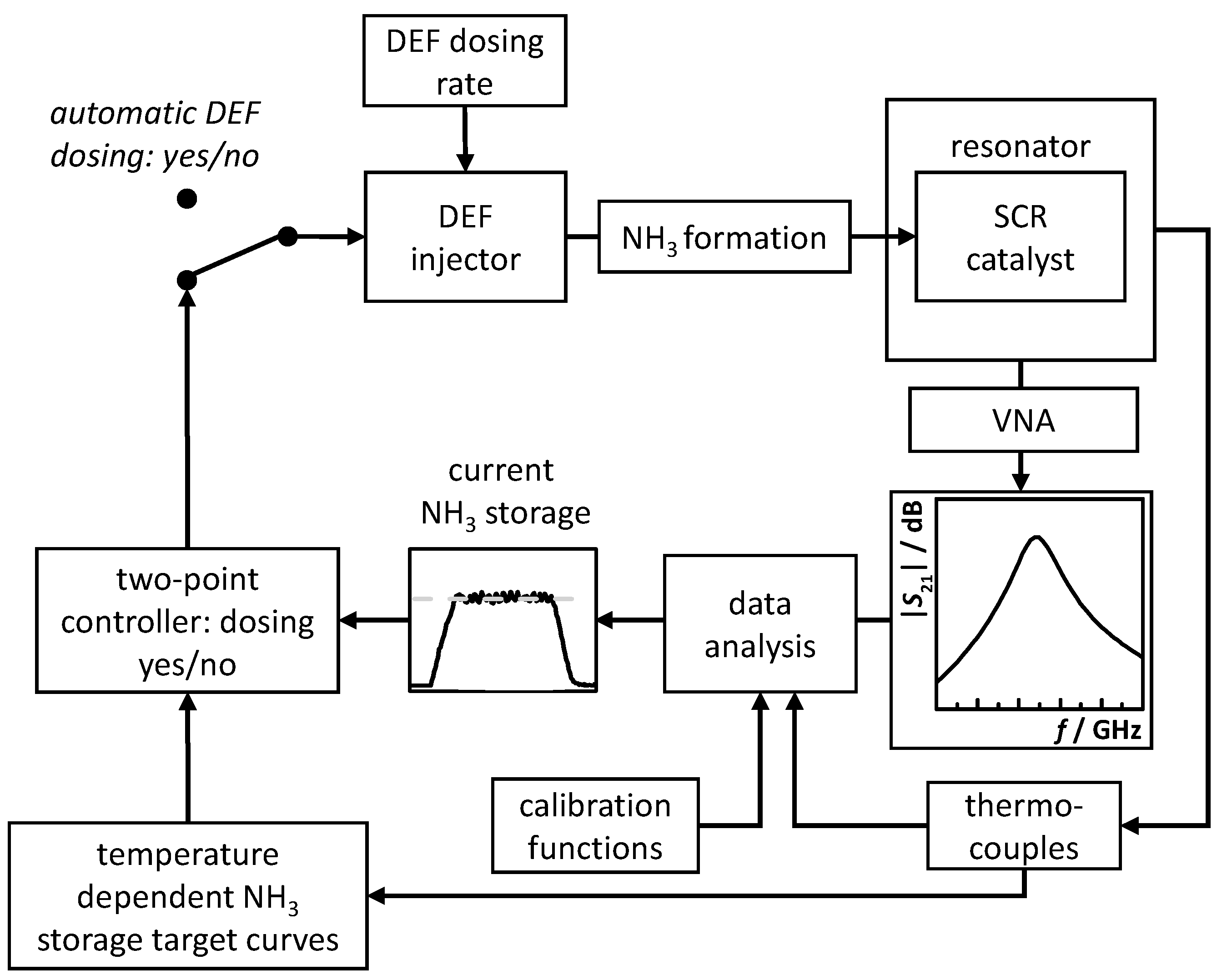
3.2. RF System Calibration, NH3 Storage Target Curves and Control Flow
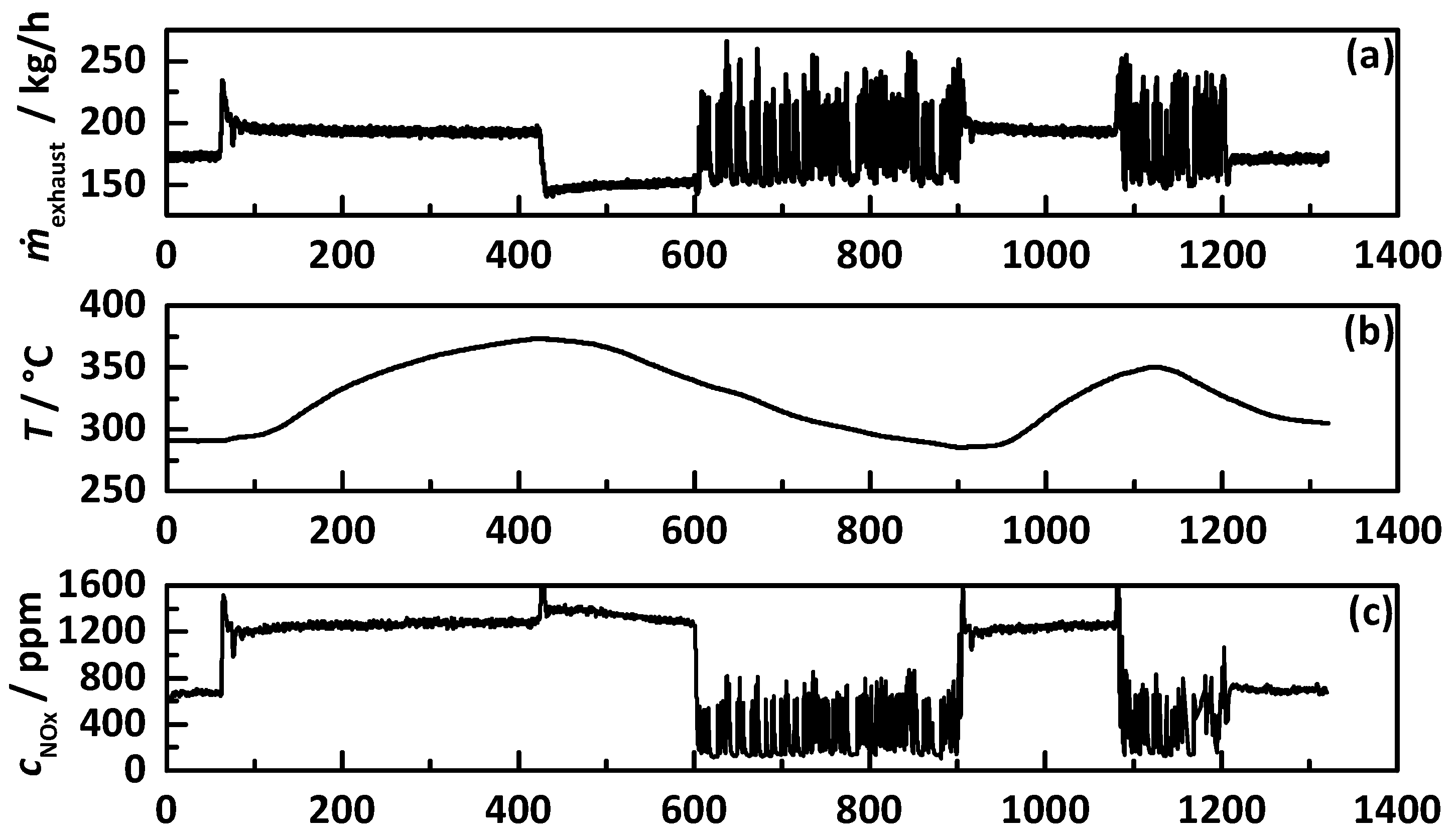
3.3. Transient Test Procedure
4. Results and Discussion
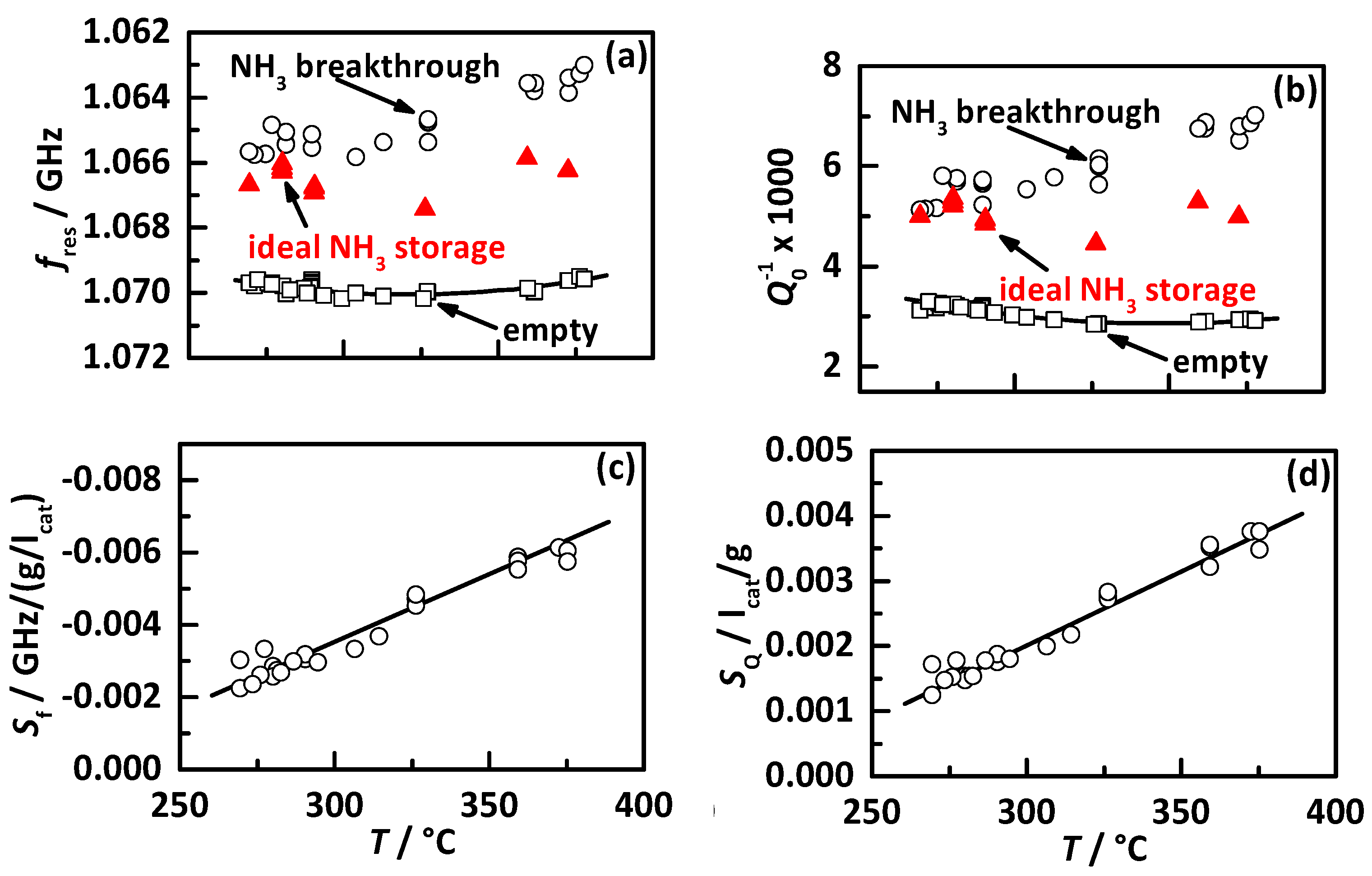
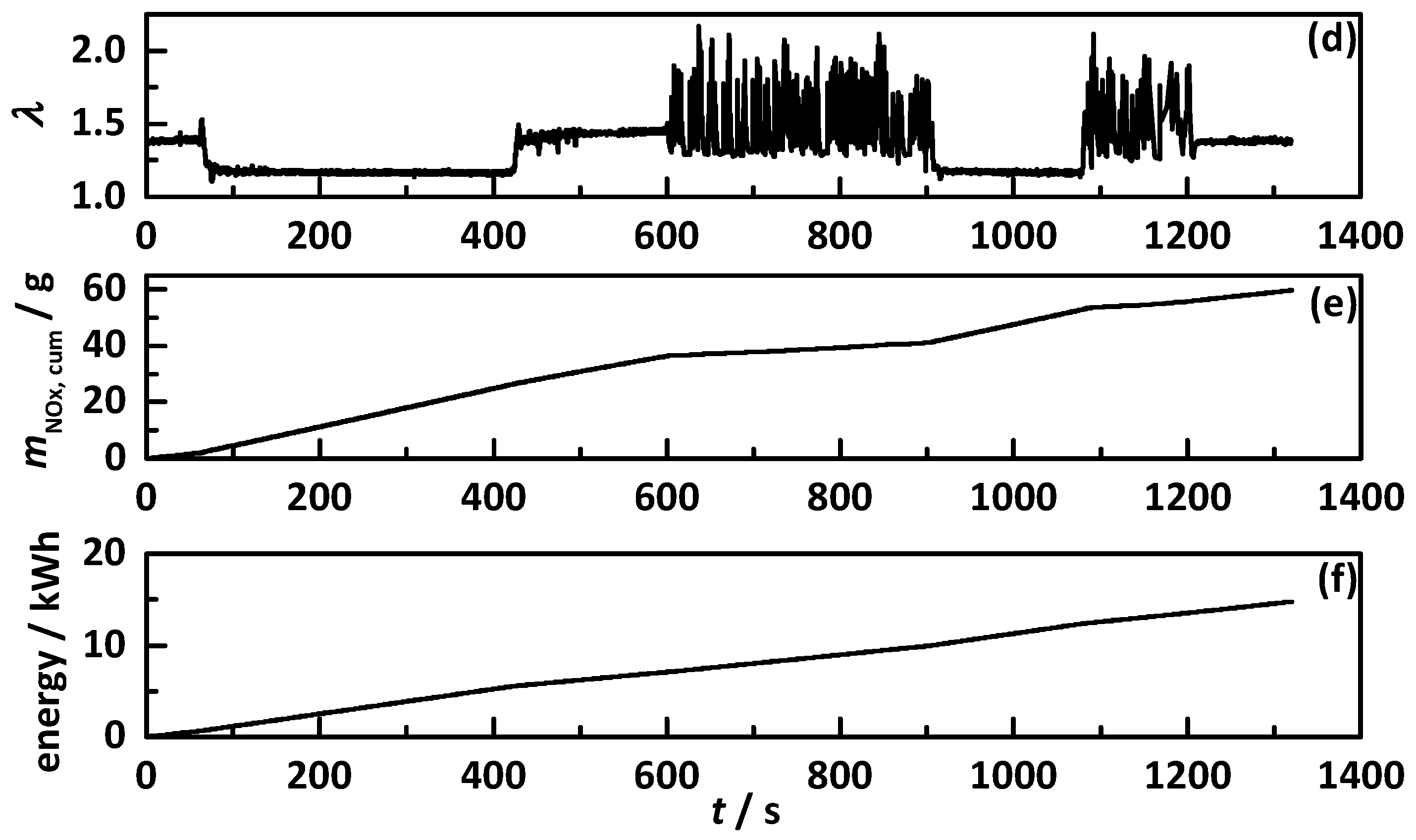
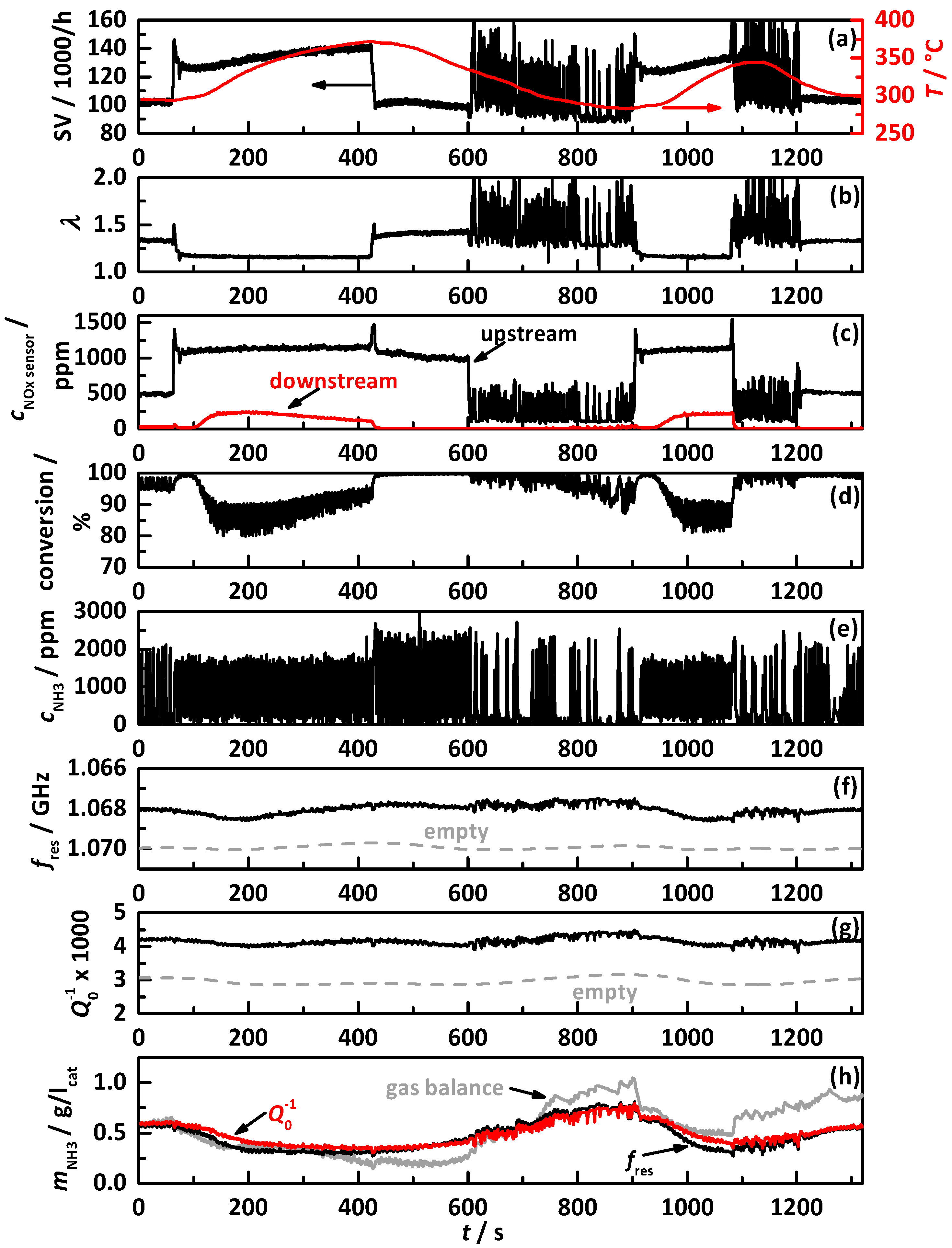
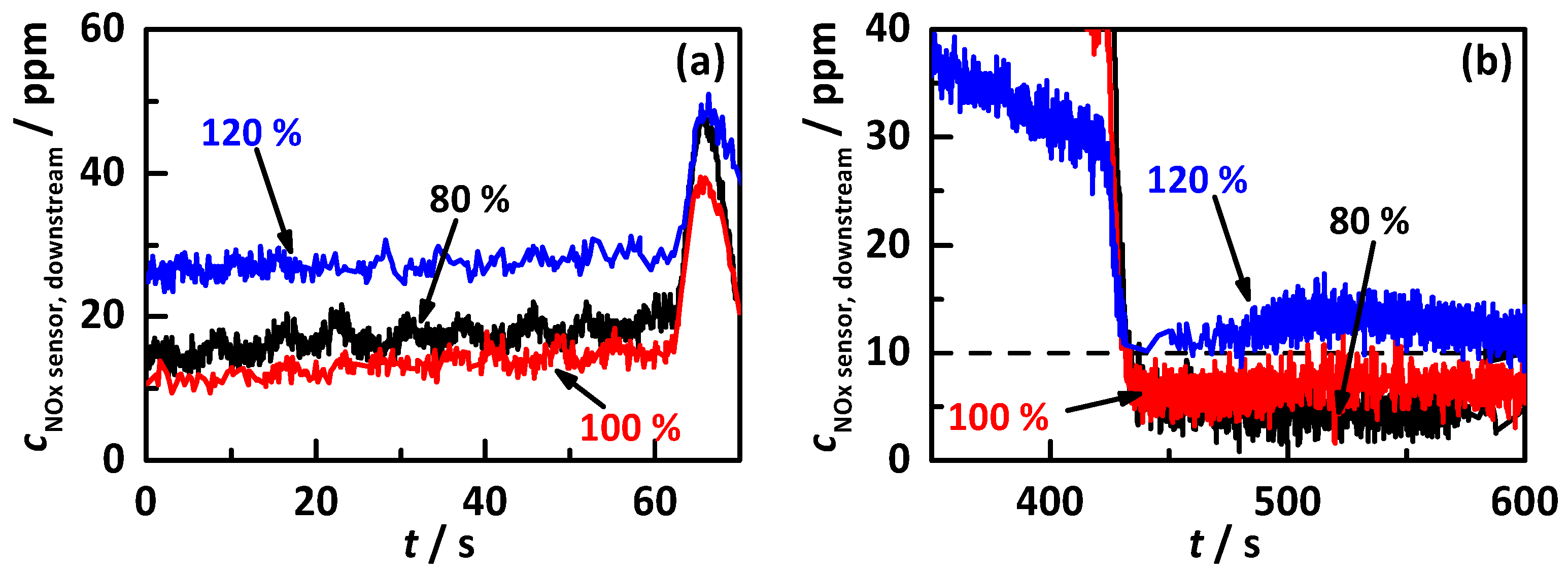
4.1. Analysis Procedure
4.2. Comparison of the Influence of Different NH3 Storage Approaches on Catalyst Performance
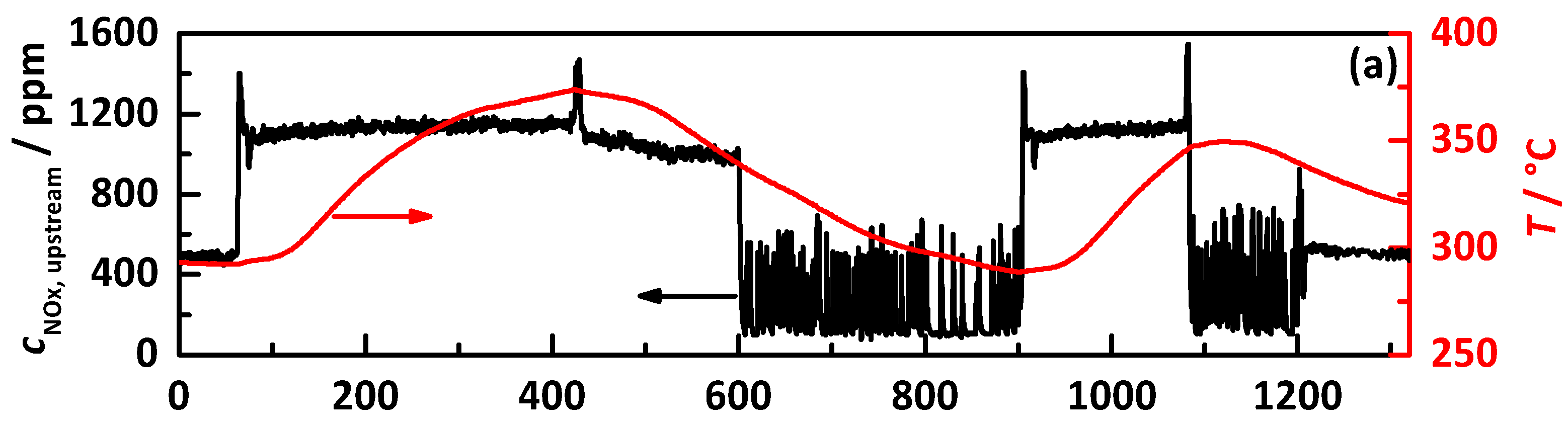
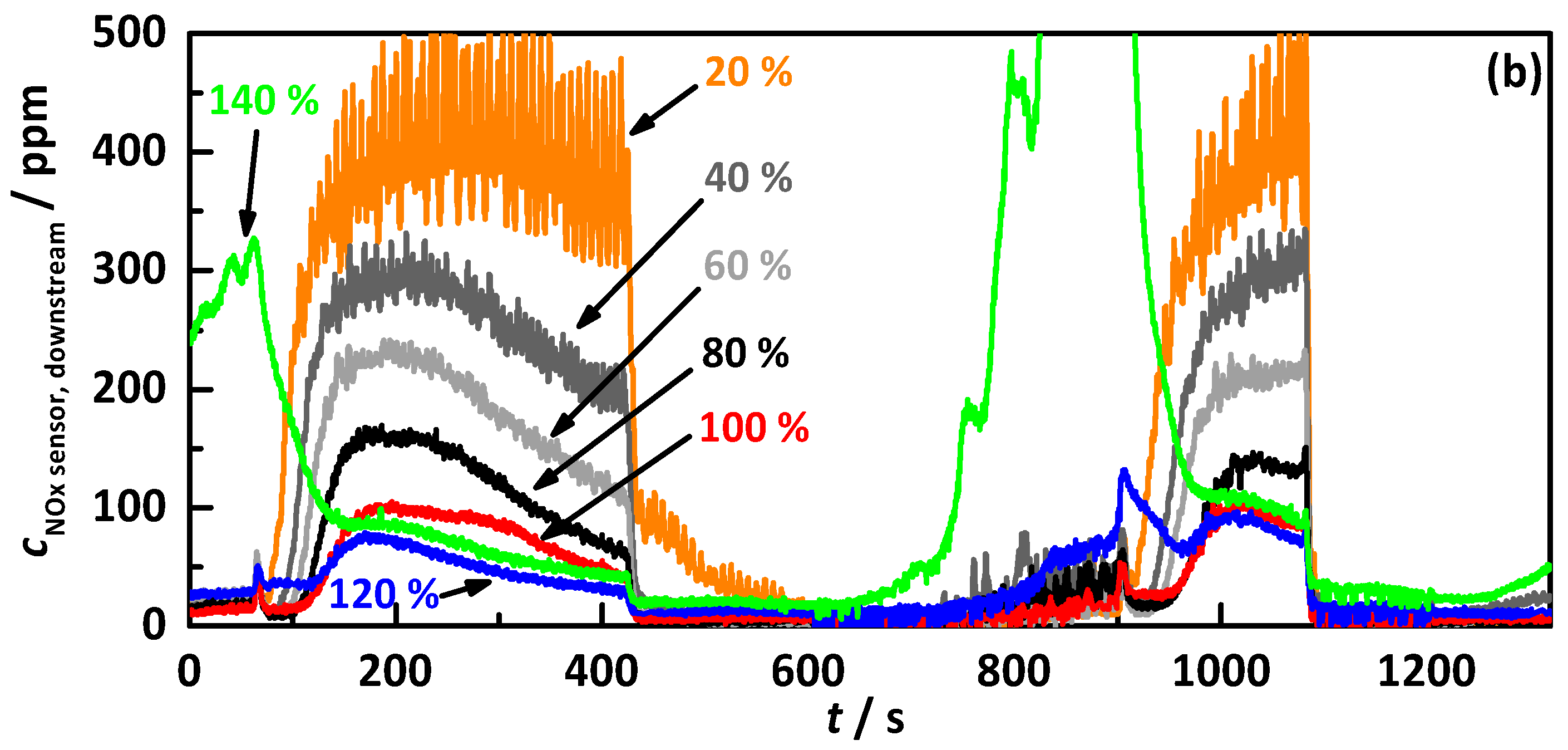
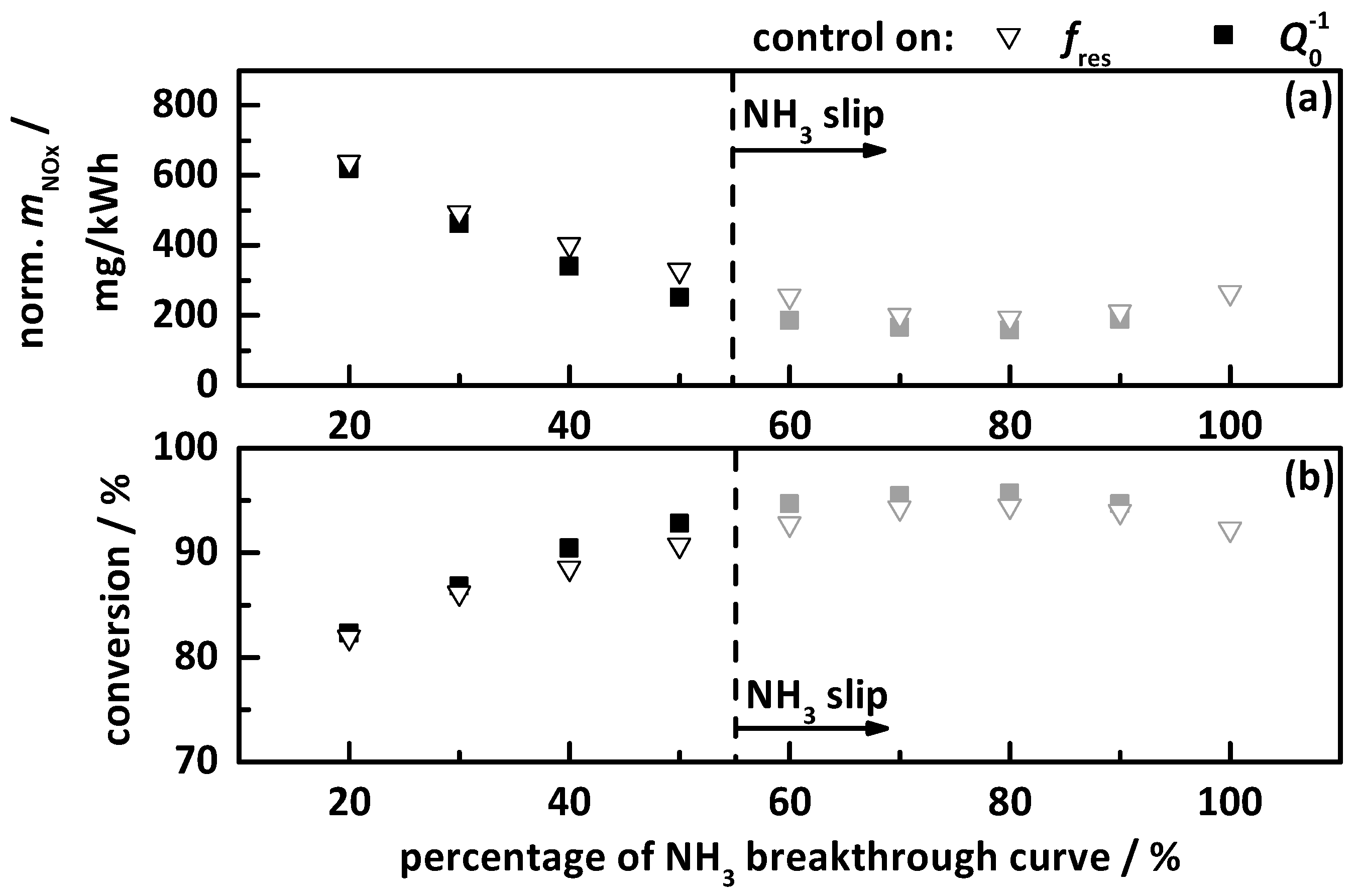
4.2.1. Control on a Percentage Gradation of the NH3 Breakthrough Curve
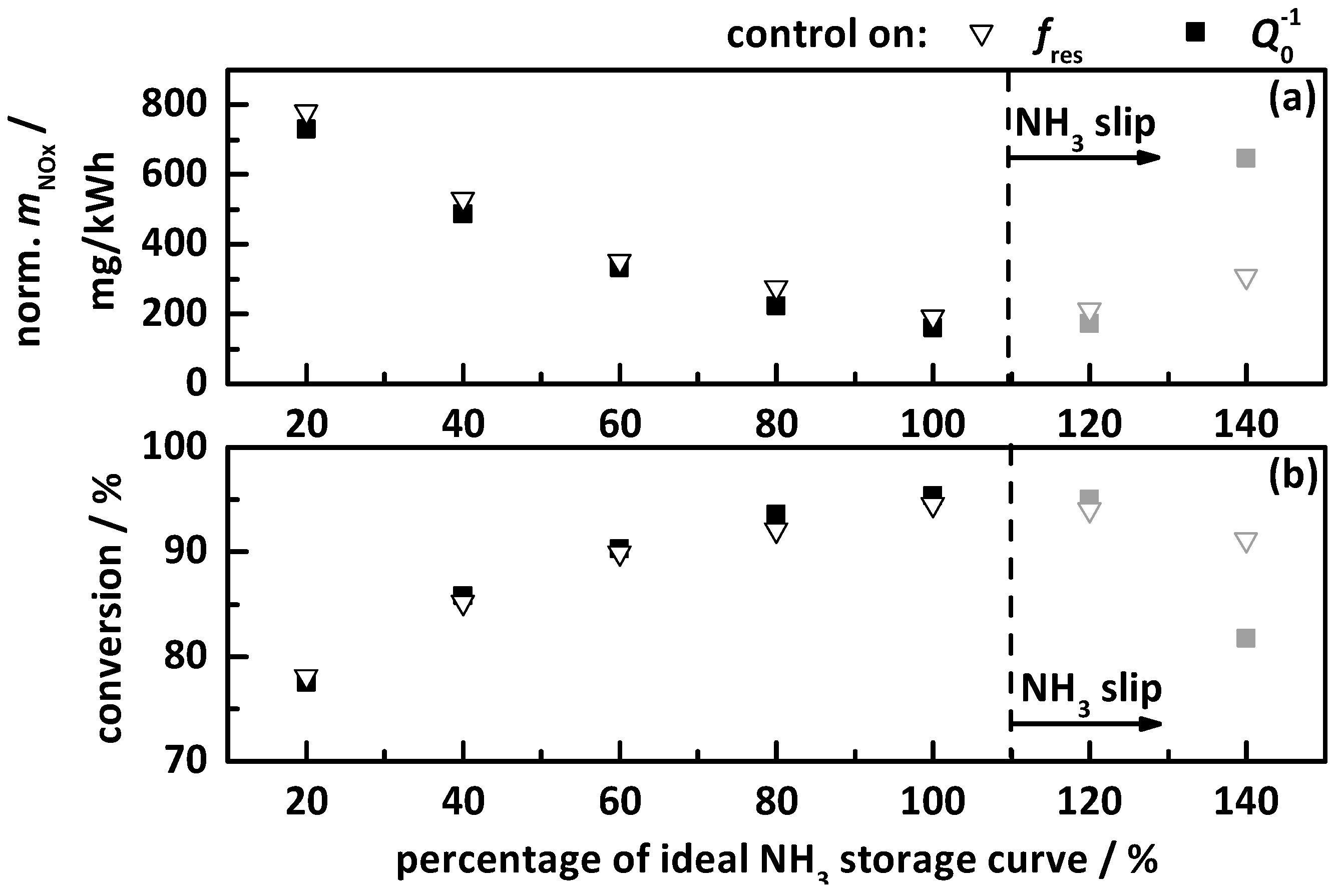
4.2.2. Control on a Percentage of the Ideal Storage Curve
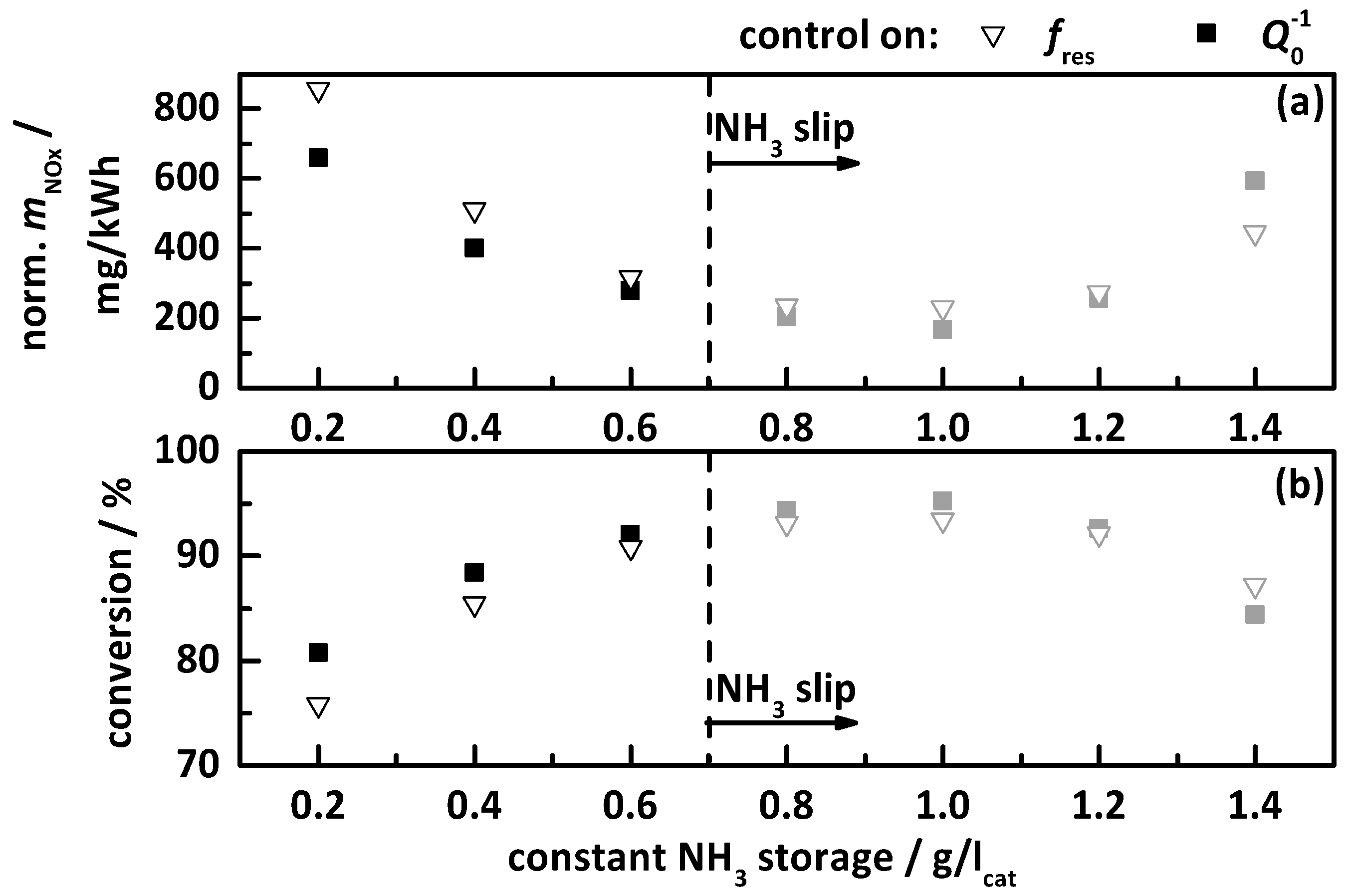
4.2.3. Control on a Constant Storage Value
5. Conclusions and Outlook
- the percentage gradation of the NH3 breakthrough storage of Ref. [20];
- the percentage gradation of the determined ideal NH3 storage curve of Ref. [20], i.e., the minimum required storage degree for maximum possible NOx conversion, and;
- a constant temperature-independent NH3 storage from 0.2 to 1.4 g/lcat.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Forzatti, P. Present status and perspectives in de-NOx SCR catalysis. Appl. Catal. A 2001, 222, 221–236. [Google Scholar] [CrossRef]
- Koebel, M.; Elsener, M.; Marti, T. NOx-Reduction in Diesel Exhaust Gas with Urea and Selective Catalytic Reduction. Combust. Sci. Tech. 1996, 121, 85–102. [Google Scholar] [CrossRef]
- Johnson, T. Review of Vehicular Emissions Trends. SAE Int. J. Engines 2015, 8, 1152–1167. [Google Scholar] [CrossRef]
- Koebel, M.; Elsener, M.; Kleemann, M. Urea-SCR: A promising technique to reduce NOx emissions from automotive diesel engines. Catal. Today 2000, 59, 335–345. [Google Scholar] [CrossRef]
- Paolucci, C.; Verma, A.A.; Bates, S.A.; Kispersky, V.F.; Miller, J.T.; Gounder, R.; Delgass, W.N.; Ribeiro, F.H.; Schneider, W.F. Isolation of the Copper Redox Steps in the Standard Selective Catalytic Reduction on Cu-SSZ-13. Angew. Chem. 2014, 126, 12022–12027. [Google Scholar] [CrossRef]
- Borfecchia, E.; Lomachenko, K.A.; Giordanino, F.; Falsig, H.; Beato, P.; Soldatov, A.V.; Bordiga, S.; Lamberti, C. Revisiting the nature of Cu sites in the activated Cu-SSZ-13 catalyst for SCR reaction. Chem. Sci. 2015, 6, 548–563. [Google Scholar] [CrossRef] [PubMed]
- Brüggemann, T.C.; Keil, F.J. Theoretical Investigation of the Mechanism of the Selective Catalytic Reduction of Nitric Oxide with Ammonia on H-Form Zeolites. J. Phys. Chem. C 2008, 112, 17378–17387. [Google Scholar] [CrossRef]
- Janssens, T.; Falsig, H.; Lundegaard, L.; Vennestrøm, P.; Rasmussen, S.; Moses, P.G.; Giordanino, F.; Borfecchia, E.; Lomachenko, K.; Lamberti, C.; et al. A Consistent Reaction Scheme for the Selective Catalytic Reduction of Nitrogen Oxides with Ammonia. ACS Catal. 2015, 5, 2832–2845. [Google Scholar] [CrossRef] [Green Version]
- Koebel, M.; Elsener, M. Selective catalytic reduction of NO over commercial DeNOx-catalysts: Experimental determination of kinetic and thermodynamic parameters. Chem. Eng. Sci. 1998, 53, 657–669. [Google Scholar] [CrossRef]
- Gao, F.; Wang, Y.; Kollar, M.; Washton, N.; Szanyi, J.; Peden, C. A comparative kinetics study between Cu/SSZ-13 and Fe/SSZ-13 SCR catalysts. Catal. Today 2015, 258, 347–358. [Google Scholar] [CrossRef]
- Guan, B.; Zhan, R.; Lin, H.; Huang, Z. Review of state of the art technologies of selective catalytic reduction of NOx from diesel engine exhaust. Appl. Therm. Eng. 2014, 66, 395–414. [Google Scholar] [CrossRef]
- Cavataio, G.; Girard, J.; Patterson, J.; Montreuil, C.; Cheng, Y.; Lambert, C. Laboratory Testing of Urea-SCR Formulations to Meet Tier 2 Bin 5 Emissions. SAE Tech. Pap. 2007, 1, 1575. [Google Scholar] [CrossRef]
- Kumar, A.; Kamasamudram, K.; Currier, N.; Yezerets, A. Effect of Transition Metal Ion Properties on the Catalytic Functions and Sulfation Behavior of Zeolite-Based SCR Catalysts. SAE Int. J. Engines 2017, 10, 1604–1612. [Google Scholar] [CrossRef]
- Yuan, X.; Liu, H.; Gao, Y. Diesel Engine SCR Control: Current Development and Future Challenges. Emiss. Control Sci. Technol. 2015, 1, 121–133. [Google Scholar] [CrossRef]
- Feng, T.; Lü, L. The characteristics of ammonia storage and the development of model-based control for diesel engine urea-SCR system. J. Ind. Eng. Chem. 2015, 28, 97–109. [Google Scholar] [CrossRef]
- Wang, D.; Yao, S.; Shost, M.; Yoo, J.; Cabush, D.; Racine, D. Ammonia Sensor for Closed-Loop SCR Control. SAE Int. J. Passenger Cars Electron. Electr. Syst. 2009, 1, 323–333. [Google Scholar] [CrossRef]
- Seibel, M.; Hofmann, F.; Christner, B.; Kaiser, A.; Fekete, N.; Weibel, M. Development and Integration Process of Control-Oriented Catalyst Models. In Proceedings of the 6th IAV MinNOx Conference, Berlin, Germany, 22–23 June 2016. [Google Scholar]
- Moser, M.; Carqué, G.; Heß, D.; Schrade, F. Simulationsgestützte Robustheitsanalyse von On-Board-Diagnosefunktionen. MTZ Motortech Z 2016, 77, 54–59. [Google Scholar] [CrossRef]
- Ning, J.; Yan, F. Detection of Injected Urea Quantity and Correction for SCR Urea Dosing Control. SAE Tech. Pap. 2015. [Google Scholar] [CrossRef]
- Dietrich, M.; Hagen, G.; Reitmeier, W.; Burger, K.; Hien, M.; Grass, P.; Kubinski, D.; Visser, J.; Moos, R. Radio-Frequency-Based NH₃-Selective Catalytic Reduction Catalyst Control: Studies on Temperature Dependency and Humidity Influences. Sensors 2017, 17, 1615. [Google Scholar] [CrossRef] [PubMed]
- Moos, R.; Rauch, D.; Votsmeier, M.; Kubinski, D. Review on Radio Frequency Based Monitoring of SCR and Three Way Catalysts. Top. Catal. 2016, 59, 961–969. [Google Scholar] [CrossRef]
- Moos, R. Microwave-Based Catalyst State Diagnosis–State of the Art and Future Perspectives. SAE Int. J. Engines 2015, 8, 1240–1245. [Google Scholar] [CrossRef]
- Moos, R.; Beulertz, G.; Reiß, S.; Hagen, G.; Fischerauer, G.; Votsmeier, M.; Gieshoff, J. Overview: Status of the Microwave-Based Automotive Catalyst State Diagnosis. Top. Catal. 2013, 56, 358–364. [Google Scholar] [CrossRef]
- Beulertz, G.; Votsmeier, M.; Moos, R. In operando Detection of Three-Way Catalyst Aging by a Microwave-Based Method: Initial Studies. Appl. Sci. 2015, 5, 174–186. [Google Scholar] [CrossRef]
- Beulertz, G.; Votsmeier, M.; Moos, R. Effect of propene, propane, and methane on conversion and oxidation state of three-way catalysts: A microwave cavity perturbation study. Appl. Catal. B 2015, 165, 369–377. [Google Scholar] [CrossRef]
- Beulertz, G.; Fritsch, M.; Fischerauer, G.; Herbst, F.; Gieshoff, J.; Votsmeier, M.; Hagen, G.; Moos, R. Microwave Cavity Perturbation as a Tool for Laboratory In Situ Measurement of the Oxidation State of Three Way Catalysts. Top. Catal. 2013, 56, 405–409. [Google Scholar] [CrossRef]
- Dietrich, M.; Jahn, C.; Lanzerath, P.; Moos, R. Microwave-Based Oxidation State and Soot Loading Determination on Gasoline Particulate Filters with Three-Way Catalyst Coating for Homogenously Operated Gasoline Engines. Sensors 2015, 15, 21971–21988. [Google Scholar] [CrossRef] [PubMed]
- Nanjundaswamy, H.; Nagaraju, V.; Wu, Y.; Koehler, E.; Sappok, A.; Ragaller, P.; Bromberg, L. Advanced RF Particulate Filter Sensing and Controls for Efficient Aftertreatment Management and Reduced Fuel Consumption. SAE Tech. Pap. 2015, 1, 0996. [Google Scholar] [CrossRef]
- Sappok, A.; Bromberg, L.; Parks, J.E.; Prikhodko, V. Loading and Regeneration Analysis of a Diesel Particulate Filter with a Radio Frequency-Based Sensor. SAE Tech. Pap. 2010, 1, 2126. [Google Scholar] [CrossRef]
- Feulner, M.; Hagen, G.; Piontkowski, A.; Müller, A.; Fischerauer, G.; Brüggemann, D.; Moos, R. In-Operation Monitoring of the Soot Load of Diesel Particulate Filters: Initial Tests. Top. Catal. 2013, 56, 483–488. [Google Scholar] [CrossRef]
- Feulner, M.; Hagen, G.; Hottner, K.; Redel, S.; Müller, A.; Moos, R. Comparative Study of Different Methods for Soot Sensing and Filter Monitoring in Diesel Exhausts. Sensors 2017, 17, 400. [Google Scholar] [CrossRef] [PubMed]
- Sappok, A.; Bromberg, L. Radio Frequency Diesel Particulate Filter Soot and Ash Level Sensors: Enabling Adaptive Controls for Heavy-Duty Diesel Applications. SAE Int. J. Commer. Veh. 2014, 7, 468–477. [Google Scholar] [CrossRef]
- Fremerey, P.; Reiß, S.; Geupel, A.; Fischerauer, G.; Moos, R. Determination of the NOx loading of an automotive lean NOx trap by directly monitoring the electrical properties of the catalyst material itself. Sensors 2011, 11, 8261–8280. [Google Scholar] [CrossRef] [PubMed]
- Moos, R.; Fischerauer, G. Automotive Catalyst State Diagnosis Using Microwaves. Oil Gas Sci. Technol. 2014, 70, 55–65. [Google Scholar] [CrossRef]
- Rauch, D.; Kubinski, D.; Cavataio, G.; Upadhyay, D.; Moos, R. Ammonia Loading Detection of Zeolite SCR Catalysts using a Radio Frequency based Method. SAE Int. J. Engines 2015, 8, 1126–1135. [Google Scholar] [CrossRef]
- Rauch, D.; Kubinski, D.; Simon, U.; Moos, R. Detection of the ammonia loading of a Cu Chabazite SCR catalyst by a radio frequency-based method. Sens. Actuator B-Chem. 2014, 205, 88–93. [Google Scholar] [CrossRef]
- Rauch, D.; Albrecht, G.; Kubinski, D.; Moos, R. A microwave-based method to monitor the ammonia loading of a vanadia-based SCR catalyst. Appl. Catal. B 2015, 165, 36–42. [Google Scholar] [CrossRef]
- Reiß, S.; Schönauer, D.; Hagen, G.; Fischerauer, G.; Moos, R. Monitoring the Ammonia Loading of Zeolite-Based Ammonia SCR Catalysts by a Microwave Method. Chem. Eng. Technol. 2011, 34, 791–796. [Google Scholar] [CrossRef]
- Dietrich, M.; Steiner, C.; Hagen, G.; Moos, R. Radio-Frequency-Based Urea Dosing Control for Diesel Engines with Ammonia SCR Catalysts. SAE Int. J. Engines 2017, 10, 1638–1645. [Google Scholar] [CrossRef]
- Chen, P.; Rauch, D.; Weide, P.; Schönebaum, S.; Simons, T.; Muhler, M.; Moos, R.; Simon, U. The effect of Cu and Fe cations on NH3 -supported proton transport in DeNOx-SCR zeolite catalysts. Catal. Sci. Technol. 2016, 6, 3362–3366. [Google Scholar] [CrossRef]
- Chen, P.; Schönebaum, S.; Simons, T.; Rauch, D.; Dietrich, M.; Moos, R.; Simon, U. Correlating the Integral Sensing Properties of Zeolites with Molecular Processes by Combining Broadband Impedance and DRIFT Spectroscopy - A New Approach for Bridging the Scales. Sensors 2015, 15, 28915–28941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rauch, D.; Dietrich, M.; Simons, T.; Simon, U.; Porch, A.; Moos, R. Microwave Cavity Perturbation Studies on H-form and Cu Ion-Exchanged SCR Catalyst Materials: Correlation of Ammonia Storage and Dielectric Properties. Top. Catal. 2017, 60, 243–249. [Google Scholar] [CrossRef]
- Dietrich, M.; Rauch, D.; Simon, U.; Porch, A.; Moos, R. Ammonia storage studies on H-ZSM-5 zeolites by microwave cavity perturbation: Correlation of dielectric properties with ammonia storage. J. Sens. Sens. Syst. 2015, 4, 263–269. [Google Scholar] [CrossRef]
- Dietrich, M.; Rauch, D.; Porch, A.; Moos, R. A laboratory test setup for in situ measurements of the dielectric properties of catalyst powder samples under reaction conditions by microwave cavity perturbation: Set up and initial tests. Sensors 2014, 14, 16856–16868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavataio, G.; Jen, H.; Warner, J.; Girard, J.; Kim, J.; Lambert, C. Enhanced Durability of a Cu/Zeolite Based SCR Catalyst. SAE Int. J. Fuels Lubr. 2009, 1, 477–487. [Google Scholar] [CrossRef]
- Moser, M.; Leimkühler, L. Simulative Assessment of Tolerance Limits of new Sensor Concepts for Controls and OBD. In Proceedings of the 4th International Specialist Conference: Sensors for Exhaust Gas Aftertreatment and CO2 Reduction, Augsburg, Germany, 27–29 June 2017. [Google Scholar]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dietrich, M.; Hagen, G.; Reitmeier, W.; Burger, K.; Hien, M.; Grass, P.; Kubinski, D.; Visser, J.; Moos, R. Radio-Frequency-Controlled Urea Dosing for NH3-SCR Catalysts: NH3 Storage Influence to Catalyst Performance under Transient Conditions. Sensors 2017, 17, 2746. https://doi.org/10.3390/s17122746
Dietrich M, Hagen G, Reitmeier W, Burger K, Hien M, Grass P, Kubinski D, Visser J, Moos R. Radio-Frequency-Controlled Urea Dosing for NH3-SCR Catalysts: NH3 Storage Influence to Catalyst Performance under Transient Conditions. Sensors. 2017; 17(12):2746. https://doi.org/10.3390/s17122746
Chicago/Turabian StyleDietrich, Markus, Gunter Hagen, Willibald Reitmeier, Katharina Burger, Markus Hien, Philippe Grass, David Kubinski, Jaco Visser, and Ralf Moos. 2017. "Radio-Frequency-Controlled Urea Dosing for NH3-SCR Catalysts: NH3 Storage Influence to Catalyst Performance under Transient Conditions" Sensors 17, no. 12: 2746. https://doi.org/10.3390/s17122746




