Molecular Imprinting Applications in Forensic Science
Abstract
:1. Introduction
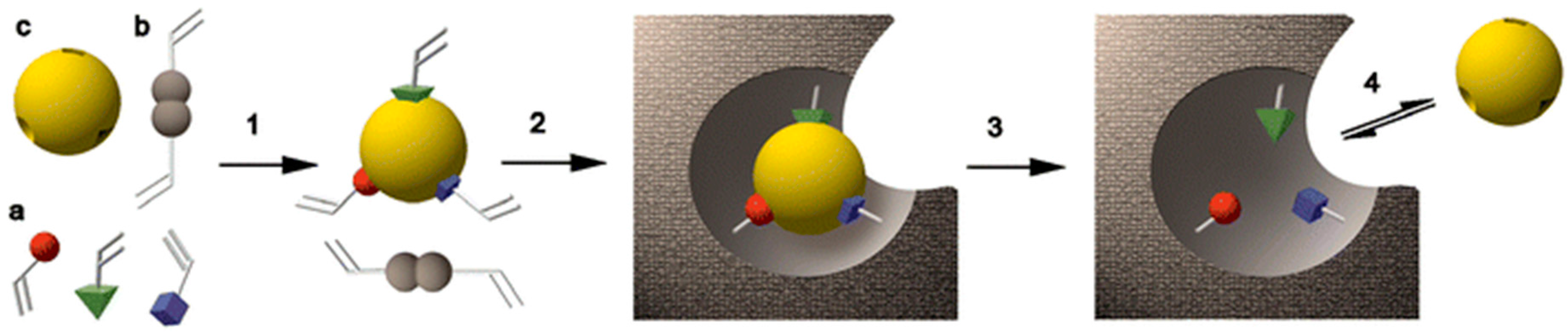
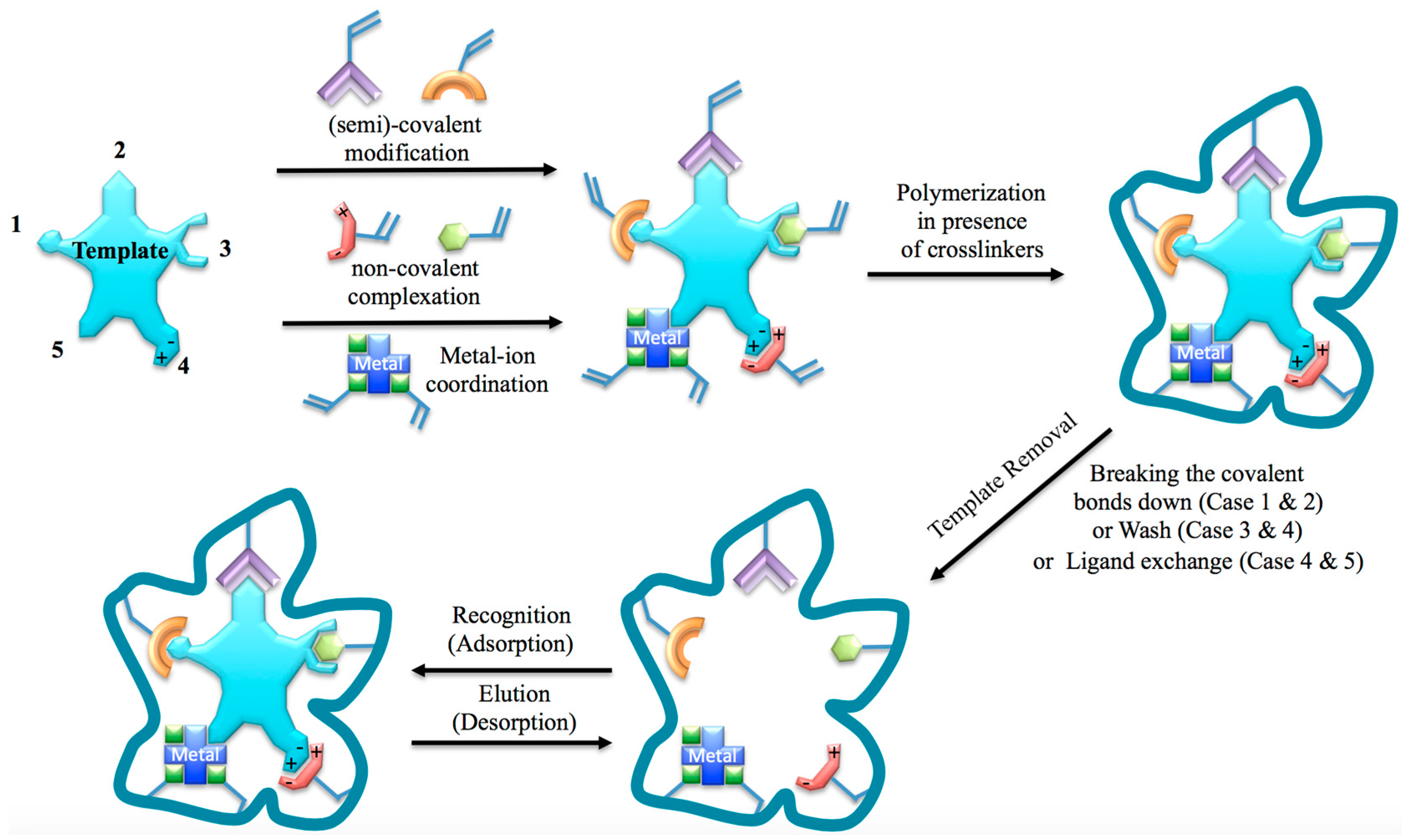
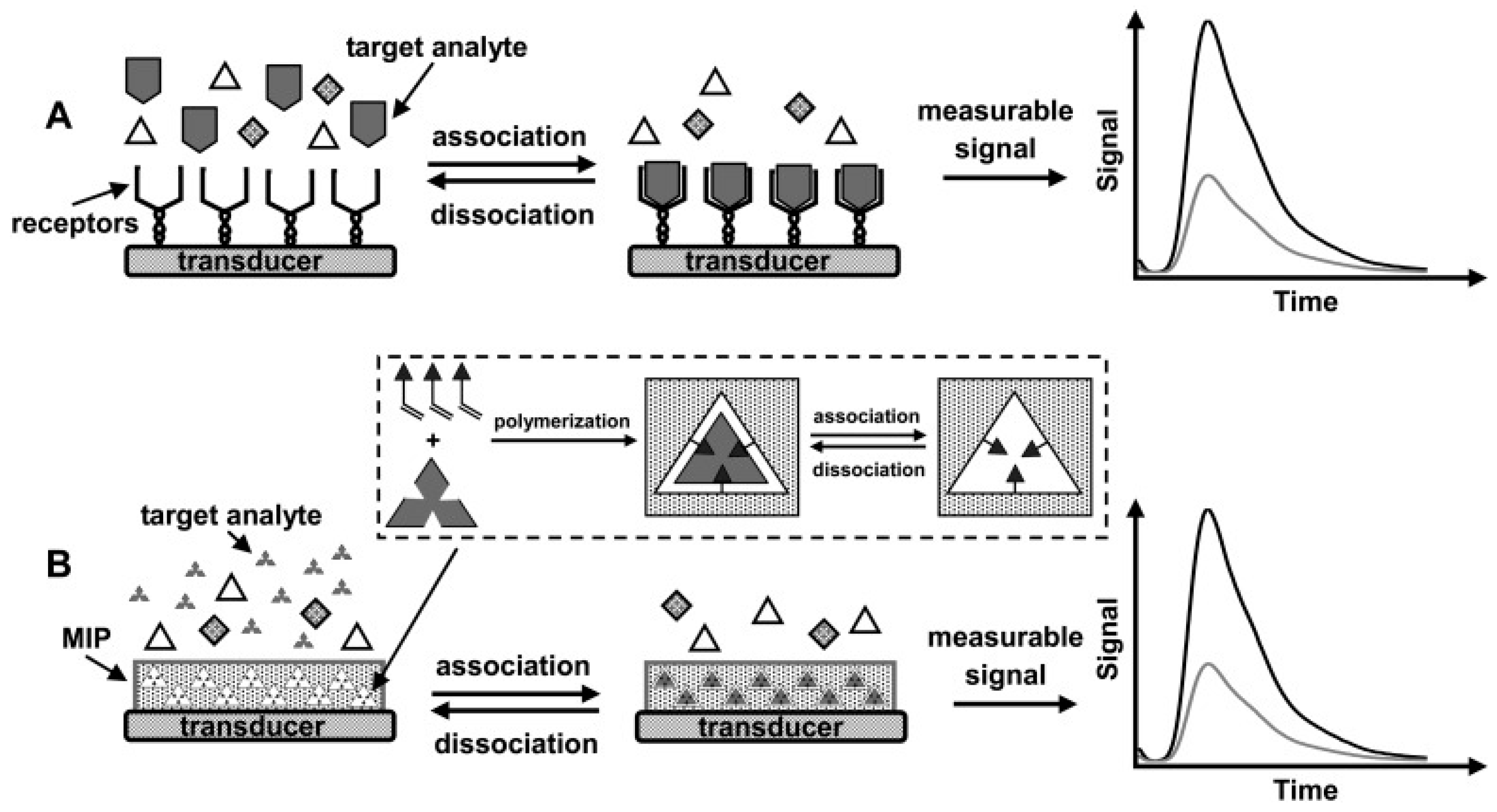
2. A Brief Theory of Molecular Imprinting
3. Imprinting Approaches for Forensic Science
3.1. Target Structures
3.2. Uses of MIPs for Pre-Concentration/Sample Preparation/Extraction
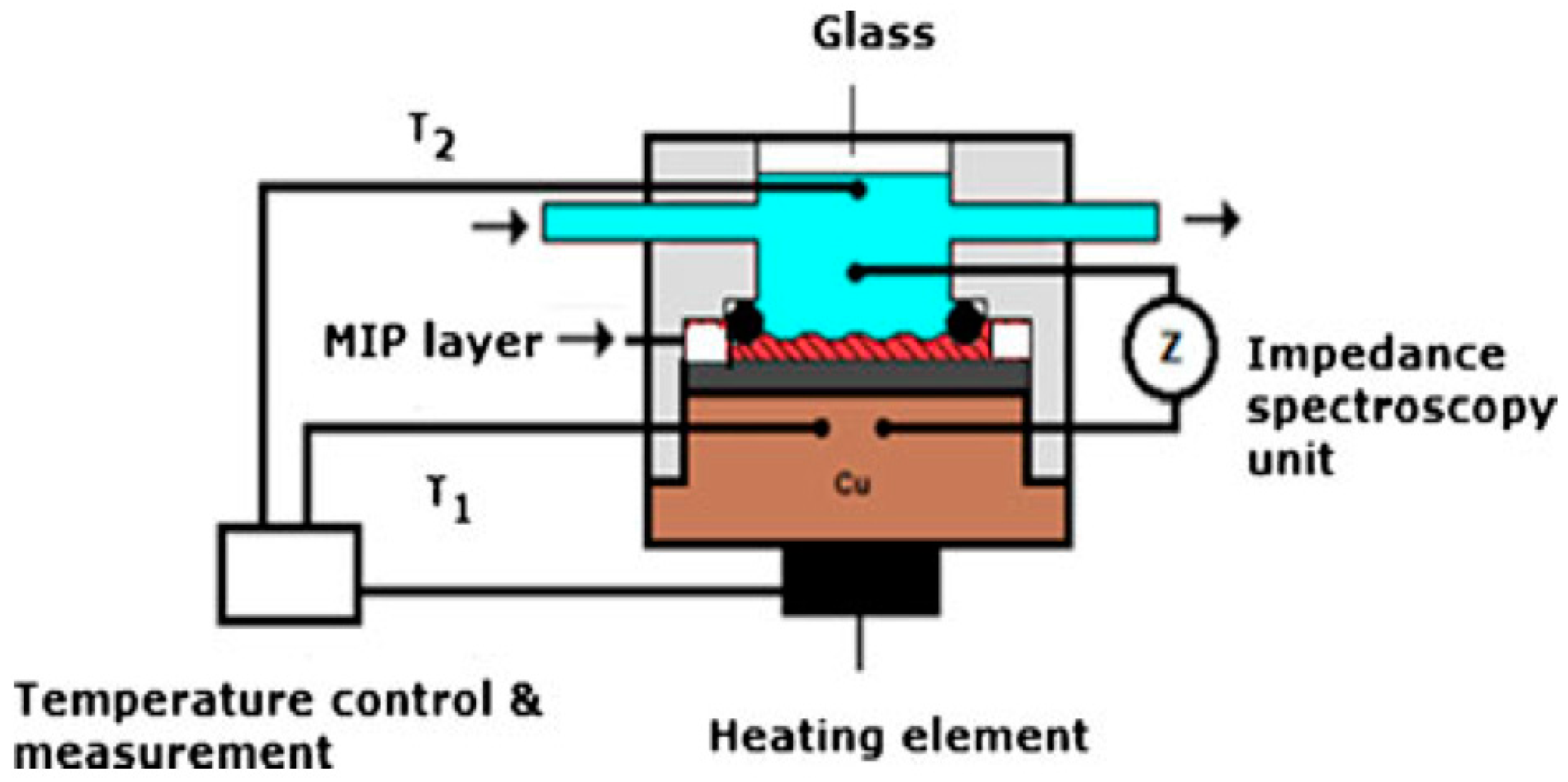
4. Instrumentation and Detection
4.1. Spectroscopy
4.2. Optical Detection
4.3. Colorimetric Sensing
4.4. Mass Detection
4.5. Electrochemical Detection
4.6. Chromatography
5. Conclusions and Future Aspects
Conflicts of Interest
References
- Uzun, L.; Turner, A.P.F. Molecularly-imprinted polymer sensors: Realising their potential. Biosens. Bioelectron. 2016, 76, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, X.; Lu, W.; Wu, X.; Li, J. Molecular imprinting: Perspectives and applications. Chem. Soc. Rev. 2016, 45, 2137–2211. [Google Scholar] [CrossRef] [PubMed]
- Bayram, E.; Yilmaz, E.; Uzun, L.; Say, R.; Denizli, A. Multiclonal plastic antibodies for selective aflatoxin extraction from food samples. Food Chem. 2017, 221, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Wulff, G.; Sarhan, A. Use of polymers with enzyme-analogous structures for the resolution of racemates. Angew. Chem. Int. Ed. 1972, 11, 341–346. [Google Scholar]
- Bui, B.T.S.; Haupt, K. Molecularly imprinted polymers: Synthetic receptors in bioanalysis. Anal. Bioanal. Chem. 2010, 398, 2481–2492. [Google Scholar]
- Ariffin, M.M.; Miller, E.I.; Cormack, P.A.; Anderson, R.A. Molecularly imprinted solid-phase extraction of diazepam and its metabolites from hair samples. Anal. Chem. 2007, 79, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.A.; Ariffin, M.M.; Cormack, P.A.G.; Miller, E.I. Comparison of molecularly imprinted solid-phase extraction (MISPE) with classical solid-phase extraction (SPE) for the detection of benzodiazepines in post-mortem hair samples. Forensic Sci. Int. 2008, 174, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, E.C.; Sparrapan, R.; Sanvido, G.B.; Santos, M.G.; Arruda, M.A.; Eberlin, M.N. Quantitation of drugs via molecularly imprinted polymer solid phase extraction and electrospray ionization mass spectrometry: Benzodiazepines in human plasma. Analyst 2011, 136, 3753–3757. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, B.; Boroujeni, M.H.; Ensafi, A.A. A novel electrochemical nanocomposite imprinted sensor for the determination of lorazepam based on modified polypyrrole@sol-gel@gold nanoparticles/pencil graphite electrode. Electrochim. Acta 2014, 123, 332–339. [Google Scholar] [CrossRef]
- Nestic, M.; Babic, S.; Pavlovic, D.M.; Sutlovic, D. Molecularly imprinted solid phase extraction for simultaneous determination of Δ9-tetrahydrocannabinol and its main metabolites by gas chromatography–mass spectrometry in urine samples. Forensic Sci. Int. 2013, 231, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Gonzalez, J.; Salgueiro-Fernandez, R.; Cabarcos, P.; Bermejo, A.M.; Bermejo-Barrera, P.; Moreda-Pineiro, A. Cannabinoids assessment in plasma and urine by high performance liquid chromatography–tandem mass spectrometry after molecularly imprinted polymer microsolid-phase extraction. Anal. Bioanal. Chem. 2017, 409, 1207–1220. [Google Scholar] [CrossRef]
- Cela-Perez, M.C.; Bates, F.; Jimenez-Morigosa, C.; Lendoiro, E.; de Castro, A.; Cruz, A.; Lopez-Rivadullab, M.; Lopez-Vilarino, J.M.; Gonzalez-Rodriguez, M.V. Water-compatible imprinted pills for sensitive determination of cannabinoids in urine and oral fluid. J. Chromatogr. A 2016, 1429, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Andersson, L.I.; Muller, R.; Vlatakis, G.; Mosbach, K. Mimics of the binding sites of opioid receptors obtained by molecular imprinting of enkephalin and morphine. Proc. Natl. Acad. Sci. USA 1995, 92, 4788–4792. [Google Scholar] [CrossRef] [PubMed]
- Piletska, E.V.; Romero-Guerra, M.; Chianella, I.; Karim, K.; Turner, A.P.F.; Piletsky, S.A. Towards the development of multisensor for drugs of abuse based on molecular imprinted polymers. Anal. Chim. Acta 2005, 542, 111–117. [Google Scholar] [CrossRef]
- Devanathan, S.; Salamon, Z.; Nagar, A.; Narang, S.; Schleich, D.; Darman, P.; Hruby, V.; Tollin, G. Subpicomolar sensing of δ-opioid receptor ligands by molecular-imprinted polymers using plasmon-waveguide resonance spectroscopy. Anal. Chem. 2005, 77, 2569–2574. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, G.; Li, M.; Huang, J.; Li, Y.; Gao, Y.; Zhang, Y. Ultrasensitive specific stimulant assay based on molecularly imprinted photonic hydrogels. Adv. Funct. Mater. 2008, 18, 575–583. [Google Scholar] [CrossRef]
- Djozan, D.; Farajzadeh, M.A.; Sorouraddin, S.M.; Baheri, T. Determination of methamphetamine, amphetamine and ecstasy by inside-needle adsorption trap based on molecularly imprinted polymer followed by GC-FID determination. Microchim. Acta 2012, 179, 209–217. [Google Scholar] [CrossRef]
- Chapuis-Hugon, F.; Cruz-Vera, M.; Savane, R.; Ali, W.H.; Valcarcel, M.; Deveaux, M.; Pichon, V. Selective sample pretreatment by molecularly imprinted polymer for the determination of LSD in biological fluids. J. Sep. Sci. 2009, 32, 3301–3309. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Shen, X.; Chaudhary, S.; Ye, L. Molecularly imprinted polymer beads prepared by Pickering emulsion polymerization for steroid recognition. J. Appl. Polym. Sci. 2014, 131, 39606. [Google Scholar] [CrossRef]
- Tan, Y.; Yin, J.; Liang, C.; Peng, H.; Nie, L.; Yao, S. A study of a new TSM bio-mimetic sensor using a molecularly imprinted polymer coating and its application for the determination of nicotine in human serum and urine. Bioelectrochemistry 2001, 53, 141–148. [Google Scholar] [CrossRef]
- Krupadam, R.J.; Venkatesh, A.; Piletsky, S.A. Molecularly imprinted polymer receptors for nicotine recognition in biological systems. Mol. Impr. 2013, 1, 27–34. [Google Scholar] [CrossRef]
- Zhou, T.; Jorgensen, L.; Mattebjerg, M.A.; Chronakis, I.S.; Ye, L. Molecularly imprinted polymer beads for nicotine recognition prepared by RAFT precipitation polymerization: A step forward towards multi-functionalities. RSC Adv. 2014, 4, 30292–30299. [Google Scholar] [CrossRef]
- Matsuguchi, M.; Uno, T. Molecular imprinting strategy for solvent molecules and its application for QCM-based VOC vapor sensing. Sens. Actuator B Chem. 2006, 113, 94–99. [Google Scholar] [CrossRef]
- Yang, J.; Hu, Y.; Cai, J.B.; Zhu, X.L.; Su, Q.D.; Hu, Y.Q.; Liang, F.X. Selective hair analysis of nicotine by molecular imprinted solid-phase extraction: An application for evaluating tobacco smoke exposure. Food Chem. Toxicol. 2007, 45, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.T.; Chen, P.Y.; Chen, J.G.; Suryanarayanan, V.; Ho, K.C. Detection of nicotine based on molecularly imprinted TiO2-modified electrodes. Anal. Chim. Acta 2009, 633, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Jan, N.; Marclay, F.; Schmutz, N.; Smith, M.; Lacoste, A.; Castella, V.; Mangin, P. Use of forensic investigations in anti-doping. Forensic Sci. Int. 2011, 213, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Ozgur, E.; Yilmaz, E.; Sener, G.; Uzun, L.; Say, R.; Denizli, A. A new molecular imprinting-based mass-sensitive sensor for real-time detection of 17β-estradiol from aqueous solution. Environ. Prog. Sustain. Energy 2013, 32, 1164–1169. [Google Scholar] [CrossRef]
- Zulfiqar, A.; Morgan, G.; Turner, N.W. Detection of multiple steroidal compounds in synthetic urine using comprehensive gas chromatography-mass spectrometry (GCxGC-MS) combined with a molecularly imprinted polymer clean-up protocol. Analyst 2014, 139, 4955–4963. [Google Scholar] [CrossRef] [PubMed]
- Kellens, E.; Bove, H.; Conradi, M.; D’Olieslaeger, L.; Wagner, P.; Landfester, K.; Ethirajan, A. Improved molecular imprinting based on colloidal particles made from miniemulsion: A case study on testosterone and its structural analogues. Macromolecules 2016, 49, 2559–2567. [Google Scholar] [CrossRef]
- Tu, X.; Muhammad, P.; Liu, J.; Ma, Y.; Wang, S.; Yin, D.; Liu, Z. Molecularly-imprinted polymer-based plasmonic immunosandwich assay for fast and ultrasensitive determination of trace glycoproteins in complex samples. Anal. Chem. 2016, 88, 12363–12370. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.; Petrikovics, I.; Lai, E.P.; Jorn, C.C. Molecularly imprinted polymer stir bar sorption extraction and electrospray ionization tandem mass spectrometry for determination of 2-aminothiazoline-4-carboxylic acid as a marker for cyanide exposure in forensic urine analysis. Anal. Method 2010, 2, 552–557. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, X.; Xu, W.; Guo, C.; Wang, S. Electrochemical sensor for the determination of brucine in human serum based on molecularly imprinted poly-o-phenylenediamine/SWNTs composite film. Sens. Actuator B Chem. 2012, 163, 84–89. [Google Scholar] [CrossRef]
- Alizadeh, T.; Rashedi, M.; Hanifehpour, Y.; Joo, S.W. Improvement of durability and analytical characteristics of arsenic-imprinted polymer-based PVC membrane electrode via surface modification of nano-sized imprinted polymer particles: Part 2. Electrochim. Acta 2015, 178, 877–885. [Google Scholar] [CrossRef]
- Nakamura, Y.; Matsunaga, H.; Haginaka, J. Preparation of molecularly imprinted polymers for strychnine by precipitation polymerization and multi-step swelling and polymerization and their application for the selective extraction of strychnine from nux-vomica extract. J. Sep. Sci. 2016, 39, 1542–1550. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Li, J.; Song, X.; Liu, J.; Lub, H.; Chen, L. Photonic and magnetic dual responsive molecularly imprinted polymers: Preparation, recognition characteristics and properties as a novel sorbent for caffeine in complicated samples. Anal. Method 2013, 5, 124–133. [Google Scholar] [CrossRef]
- Ogiso, M.; Minoura, N.; Shinbo, T.; Shimizu, T. Detection of a specific DNA sequence by electrophoresis through a molecularly imprinted polymer. Biomaterials 2006, 27, 4177–4182. [Google Scholar] [CrossRef] [PubMed]
- Emir Diltemiz, S.; Hur, D.; Ersoz, A.; Denizli, A.; Say, R. Designing of MIP based QCM sensor having thymine recognition sites based on biomimicking DNA approach. Biosens. Bioelectron. 2009, 25, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Ersoz, A.; Emir Diltemiz, S.; Atilir Ozcan, A.; Denizli, A.; Say, R. 8-OHdG sensing with MIP based solid phase extraction and QCM technique. Sens. Actuator B Chem. 2009, 137, 7–11. [Google Scholar] [CrossRef]
- Uzek, R.; Uzun, L.; Senel, S.; Denizli, A. Nanospines incorporation into the structure of the hydrophobic cryogels via novel cryogelation method: An alternative sorbent for plasmid DNA purification. Coll. Surf. B Biointerface 2013, 102, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Zhang, Z.; Wu, M.; Xie, C.; Guan, G.; Wang, D. A surface functional monomer-directing strategy for highly dense imprinting of TNT at surface of silica nanoparticles. J. Am. Chem. Soc. 2007, 129, 7859–7866. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.; Zhang, Z.; Wang, Z.; Liu, B.; Gao, D.; Xie, C. Single-hole hollow polymer microspheres toward specific high-capacity uptake of target species. Adv. Mater. 2007, 19, 2370–2374. [Google Scholar] [CrossRef]
- Li, J.; Kendig, C.E.; Nesterov, E.E. Chemosensory performance of molecularly imprinted fluorescent conjugated polymer materials. J. Am. Chem. Soc. 2007, 129, 15911–15918. [Google Scholar] [CrossRef] [PubMed]
- Holthoff, E.L.; Stratis-Cullum, D.N.; Hankus, M.E. A nanosensor for TNT detection based on molecularly imprinted polymers and surface enhanced Raman scattering. Sensors 2011, 11, 2700–2714. [Google Scholar] [CrossRef] [PubMed]
- Riskin, M.; Tel-Vered, R.; Willner, I. Imprinted Au-nanoparticle composites for the ultrasensitive surface plasmon resonance detection of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX). Adv. Mater. 2010, 22, 1387–1391. [Google Scholar] [CrossRef] [PubMed]
- Riskin, M.; Ben-Amram, Y.; Tel-Vered, R.; Chegel, V.; Almog, J.; Willner, I. Molecularly imprinted Au nanoparticles composites on au surfaces for the surface plasmon resonance detection of pentaerythritol tetranitrate, nitroglycerin, and ethylene glycol dinitrate. Anal. Chem. 2011, 83, 3082–3088. [Google Scholar] [CrossRef] [PubMed]
- Lordel, S.; Chapuis-Hugon, F.; Eudes, V.; Pichon, V. Development of imprinted materials for the selective extraction of nitroaromatic explosives. J. Chromatogr. A 2010, 1217, 6674–6680. [Google Scholar] [CrossRef] [PubMed]
- Lordel, S.; Chapuis-Hugon, F.; Eudes, V.; Pichon, V. Selective extraction of nitroaromatic explosives by using molecularly imprinted silica sorbents. Anal. Bioanal. Chem. 2011, 399, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Lordel-Madeleine, S.; Eudes, V.; Pichon, V. Identification of the nitroaromatic explosives in post-blast samples by online solid phase extraction using molecularly imprinted silica sorbent coupled with reversed-phase chromatography. Anal. Bioanal. Chem. 2013, 405, 5237–5247. [Google Scholar] [CrossRef] [PubMed]
- Mamo, S.K.; Gonzalez-Rodriguez, J. Development of a molecularly imprinted polymer-based sensor for the electrochemical determination of triacetone triperoxide (TATP). Sensors 2014, 14, 23269–23282. [Google Scholar] [CrossRef] [PubMed]
- Goudsmits, E.; Sharples, G.P.; Birkett, J.W. Recent trends in organic gunshot residue analysis. Trends Anal. Chem. 2015, 74, 46–57. [Google Scholar] [CrossRef]
- Taudte, R.V.; Beavis, A.; Blanes, L.; Cole, N.; Doble, P.; Roux, C. Detection of gunshot residues using mass spectrometry. Biomed. Res. Int. 2014, 2014, 965403. [Google Scholar] [CrossRef] [PubMed]
- Pereira, E.; Caceres, C.; Rivera, F.; Rivas, B.; Saez, P. Preparation of molecularly imprinted polymers for diphenylamine removal from organic gunshot residues. J. Chilean Chem. Soc. 2014, 59, 2731–2736. [Google Scholar] [CrossRef]
- Kabir, A.; Holness, H.; Furton, K.G.; Almirall, J.R. Recent advances in micro-sample preparation with forensic applications. Trends Anal. Chem. 2013, 45, 264–279. [Google Scholar] [CrossRef]
- Alizadeh, T.; Rezaloo, F. A new chemiresistor sensor based on a blend of carbon nanotube, nano-sized molecularly imprinted polymer and poly methyl methacrylate for the selective and sensitive determination of ethanol vapor. Sens. Actuator B Chem. 2013, 176, 28–37. [Google Scholar] [CrossRef]
- Boyd, J.W.; Cobb, G.P.; Southard, G.E.; Murray, G.M. Development of molecularly imprinted polymer sensors for chemical warfare agents. Johns Hopkins APL Tech. Dig. 2004, 25, 44–49. [Google Scholar]
- Prathish, K.P.; Prasad, K.; Rao, T.P.; Suryanarayana, M.V.S. Molecularly imprinted polymer-based potentiometric sensor for degradation product of chemical warfare agents: Part I. Methylphosphonic acid. Talanta 2007, 71, 1976–1980. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Xue, M.; Xu, Z.; Dong, X.; Xue, F.; Wang, F.; Wang, Q.; Meng, Z. Molecularly imprinted polymers for the sensing of explosives and chemical warfare agents. Curr. Org. Chem. 2015, 19, 62–71. [Google Scholar] [CrossRef]
- Mudge, S.M. Environmental forensics and the importance of source identification, Issues in environmental science and technology, No. 26. Environ. Forensics 2008. [Google Scholar] [CrossRef]
- Davis, A.; Howe, B.; Nicholson, A.; McCaffery, S.; Hoenke, K.A. Use of geochemical forensics to determine release eras of petrochemicals to groundwater, Whitehorse, Yukon. Environ. Forensics 2005, 6, 253–271. [Google Scholar] [CrossRef]
- Alizadeh, T.; Zare, M.; Ganjali, M.R.; Norouzi, P.; Tavana, B. A new molecularly imprinted polymer (MIP)-based electrochemical sensor for monitoring 2,4,6-trinitrotoluene (TNT) in natural waters and soil samples. Biosens. Bioelectron. 2010, 25, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Sellergren, B.; Allender, C.J. Molecularly imprinted polymers: A bridge to advanced drug delivery. Adv. Drug Deliv. Rev. 2005, 57, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cao, S.; Piletsky, S.A.; Turner, A.P.F. Molecularly Imprinted Catalysts, Principles: Syntheses, and Applications, 1st ed.; Elsevier: Waltham, MA, USA, 2016. [Google Scholar]
- Corman, M.E.; Armutcu, C.; Uzun, L.; Denizli, A. Cryogel based molecularly imprinted composite cartridges for rapid, efficient and selective preconcentration of polycyclic aromatic hydrocarbons (PAHs) from water samples through hydrophobic interactions. Mater. Sci. Eng. C 2017, 70, 41–53. [Google Scholar]
- Erol, K.; Kose, K.; Uzun, L.; Say, R.; Denizli, A. Polyethyleneimine assisted-two-step polymerization to develop surface imprinted cryogels for lysozyme purification. Coll. Surf. B Biointerface 2016, 146, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Ozaydin Ince, G.; Armagan, E.; Erdogan, H.; Buyukserin, F.; Uzun, L.; Demirel, G. One-dimensional surface-imprinted polymeric nanotubes for specific biorecognition by initiated chemical vapor deposition (iCVD). ACS Appl. Mater. Interface 2013, 5, 6447–6452. [Google Scholar] [CrossRef] [PubMed]
- Osman, B.; Uzun, L.; Besirli, N.; Denizli, A. Microcontact imprinted surface plasmon resonance sensor for myoglobin detection. Mater. Sci. Eng. C 2013, 33, 3609–3614. [Google Scholar] [CrossRef] [PubMed]
- Sener, G.; Ozgur, E.; Rad, A.Y.; Uzun, L.; Say, R.; Denizli, A. Rapid real-time detection of procalcitonin using a microcontact imprinted surface plasmon resonance biosensor. Analyst 2013, 138, 6422–6428. [Google Scholar] [CrossRef] [PubMed]
- Zander, A.; Findlay, P.; Renner, T.; Sellergren, B.; Swietlow, A. Analysis of nicotine and its oxidation products in nicotine chewing gum by a molecularly imprinted solid-phase extraction. Anal. Chem. 1998, 70, 3304–3314. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.S.; D’Souza, F.; Kutner, W. Molecular imprinting for selective chemical sensing of hazardous compounds and drugs of abuse. Trends Anal. Chem. 2012, 34, 59–77. [Google Scholar] [CrossRef]
- Guan, G.; Liu, B.; Wang, Z.; Zhang, Z. Imprinting of molecular recognition sites on nanostructures and its applications in chemosensors. Sensors 2008, 8, 8291–8320. [Google Scholar] [CrossRef] [PubMed]
- Karpas, Z. Ion Mobility Spectrometry in Forensic Science. Encycl. Anal. Chem. 2006. [Google Scholar] [CrossRef]
- Jafari, M.T.; Rezaei, B.; Zaker, B. Ion mobility spectrometry as a detector for molecular imprinted polymer separation and metronidazole determination in pharmaceutical and human serum samples. Anal. Chem. 2009, 81, 3585–3591. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, B.; Jafari, M.T.; Khademi, R. Selective separation and determination of primidone in pharmaceutical and human serum samples using molecular imprinted polymer-electrospray ionization ion mobility spectrometry (MIP-ESI-IMS). Talanta 2009, 79, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Li, H.Y.; Meng, Z.H.; Liang, X.X.; Xue, M.; Wang, Q.H.; Dong, X. Detection of nitrobenzene compounds in surface water by ion mobility spectrometry coupled with molecularly imprinted polymers. J. Hazard. Mater. 2014, 280, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Homola, J. Surface plasmon resonance sensors for detection of chemical and biological species. Chem. Rev. 2008, 108, 462–493. [Google Scholar] [CrossRef] [PubMed]
- Uzun, L.; Say, R.; Unal, S.; Denizli, A. Production of surface plasmon resonance based assay kit for hepatitis diagnosis. Biosens. Bioelectron. 2009, 24, 2878–2884. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, E.; Majidi, D.; Ozgur, E.; Denizli, A. Whole cell imprinting based Escherichia coli sensors: A study for SPR and QCM. Sens. Actuator B Chem. 2015, 209, 714–721. [Google Scholar] [CrossRef]
- Hu, X.; Li, G.; Huang, J.; Zhang, D.; Qiu, Y. Construction of self-reporting specific chemical sensors with high sensitivity. Adv. Mater. 2007, 19, 4327–4332. [Google Scholar] [CrossRef]
- Meng, L.; Meng, P.; Tang, B.; Zhang, Q.; Wang, Y. Molecularly imprinted photonic hydrogels for fast screening of atropine in biological samples with high sensitivity. Forensic Sci. Int. 2013, 231, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Taranekar, P.; Huang, C.Y.; Advincula, R.C. Pinacolyl methyl phosphonate (PMP) detection by molecularly imprinted polymers (MIP): A labile covalent bonding approach. Polymer 2006, 47, 6485–6490. [Google Scholar] [CrossRef]
- Meng, L.; Meng, P.; Zhang, Q.; Wang, Y. Water-compatible molecularly imprinted photonic hydrogels for fast screening of morphine in urine. Chin. J. Anal. Chem. 2015, 43, 490–496. [Google Scholar]
- Lu, W.; Xue, F.; Huang, S.Y.; Meng, Z.H.; Xue, M. Molecularly imprinted colloidal array for detection of explosives. Chin. J. Anal. Chem. 2012, 40, 1561–1566. [Google Scholar] [CrossRef]
- Han, C.; Shang, Z.; Zhang, H.; Song, Q. Detection of hidden drugs with a molecularly imprinted electrochemiluminescence sensor. Anal. Method 2013, 5, 6064–6070. [Google Scholar] [CrossRef]
- Liu, M.; Lu, J.; He, Y.; Du, J. Molecular imprinting–chemiluminescence sensor for the determination of brucine. Anal. Chim. Acta 2005, 541, 97–102. [Google Scholar] [CrossRef]
- Lulka, M.F.; Iqbal, S.S.; Chambers, J.P.; Valdes, E.R.; Thompson, R.G.; Goode, M.T.; Valdes, J.J. Molecular imprinting of Ricin and its A and B chains to organic silanes: Fluorescence detection. Mater. Sci. Eng. C 2000, 11, 101–105. [Google Scholar] [CrossRef]
- Stringer, R.C.; Gangopadhyay, S.; Grant, S.A. Detection of nitroaromatic explosives using a fluorescent-labeled imprinted polymer. Anal. Chem. 2010, 82, 4015–4019. [Google Scholar] [CrossRef] [PubMed]
- Stringer, R.C.; Gangopadhyay, S.; Grant, S.A. Comparison of molecular imprinted particles prepared using precipitation polymerization in water and chloroform for fluorescent detection of nitroaromatics. Anal. Chim. Acta 2011, 703, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Chianella, I.; Piletsky, S.A.; Tothill, I.E.; Chen, B.; Turner, A.P.F. MIP-based solid phase extraction cartridges combined with MIP-based sensors for the detection of microcystin-LR. Biosens. Bioelectron. 2003, 18, 119–127. [Google Scholar] [CrossRef]
- Percival, C.J.; Stanley, S.; Braithwaite, A.; Newton, M.I.; McHale, G. Molecular imprinted polymer coated QCM for the detection of nandrolone. Analyst 2002, 127, 1024–1026. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Yang, L.; Mu, N.; Shao, S.; Wang, W.; Xie, X.; He, S. A SAW-based chemical sensor for detecting sulfur-containing organophosphorus compounds using a two-step self-assembly and molecular imprinting technology. Sensors 2014, 14, 8810–8820. [Google Scholar] [CrossRef] [PubMed]
- Tanada, N.; Kashimura, S.; Kageura, M.; Hara, K. Utility of caffeine analysis for forensic hair discrimination. Jpn. J. Legal Med. 1998, 52, 233–237. [Google Scholar] [CrossRef]
- Liang, C.; Peng, H.; Bao, X.; Nie, L.; Yao, S. Study of a molecular imprinting polymer coated BAW bio-mimic sensor and its application to the determination of caffeine in human serum and urine. Analyst 1999, 124, 1781–1785. [Google Scholar] [CrossRef] [PubMed]
- Uzun, L.; Tiwari, A. Advanced Imprinting Materials; Wiley-Scrivener: New York, NY, USA, 2016. [Google Scholar]
- Deng, D.L.; Zhang, J.Y.; Chen, C.; Hou, X.L.; Su, Y.Y.; Wu, L. Monolithic molecular imprinted polymer fiber for recognition and solid phase microextraction of ephedrine and pseudoephedrine in biological samples prior to capillary electrophoresis analysis. J. Chromatogr. A 2012, 1219, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Peeters, M.; Csipai, P.; Geerets, B.; Weustenraed, A.; van Grinsven, B.; Thoelen, R.; Gruber, J.; De Ceuninck, W.; Cleij, T.J.; Troost, F.J.; et al. Heat-transfer-based detection of L-nicotine, histamine, and serotonin using molecularly imprinted polymers as biomimetic receptors. Anal. Bioanal. Chem. 2013, 405, 6453–6460. [Google Scholar] [CrossRef] [PubMed]
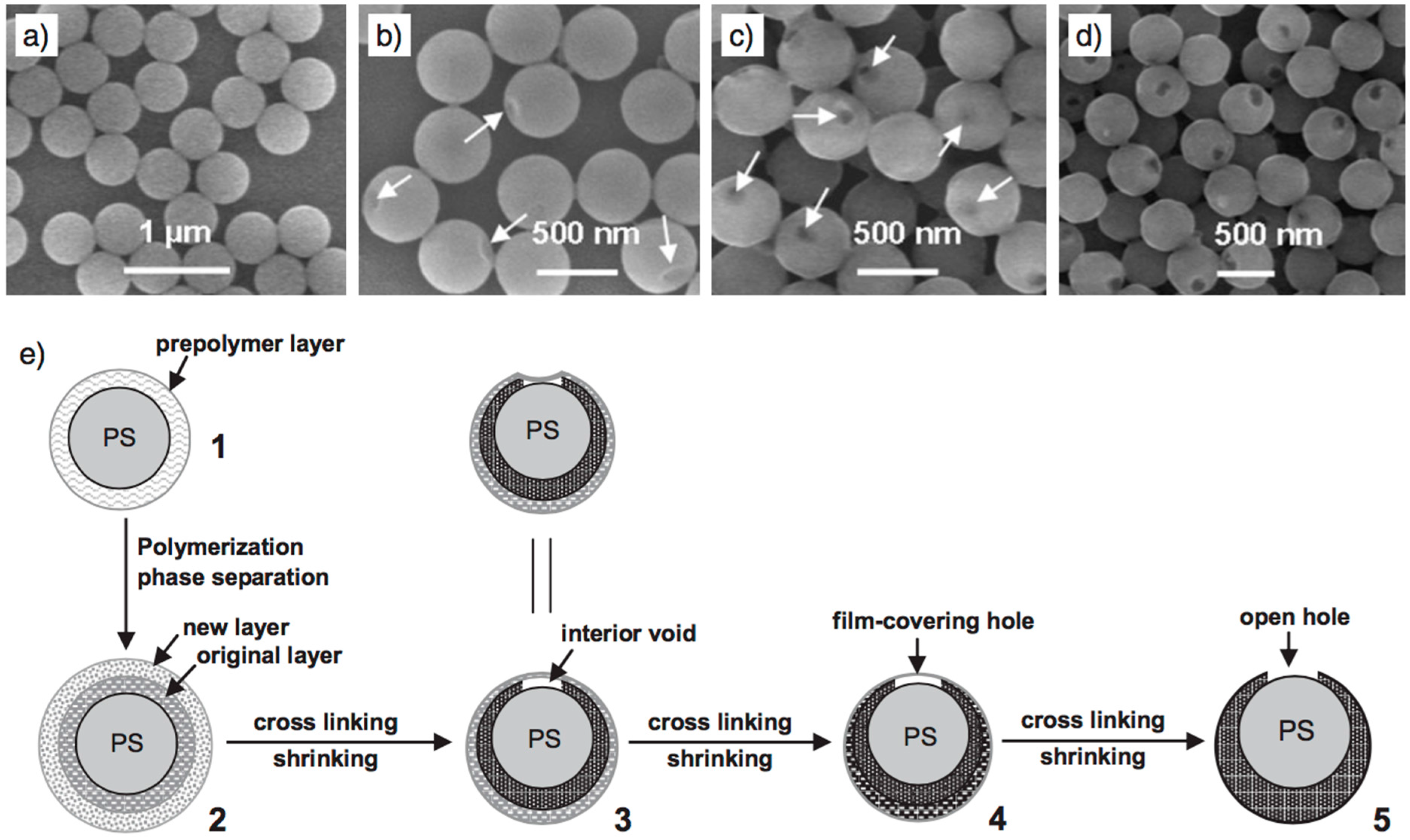
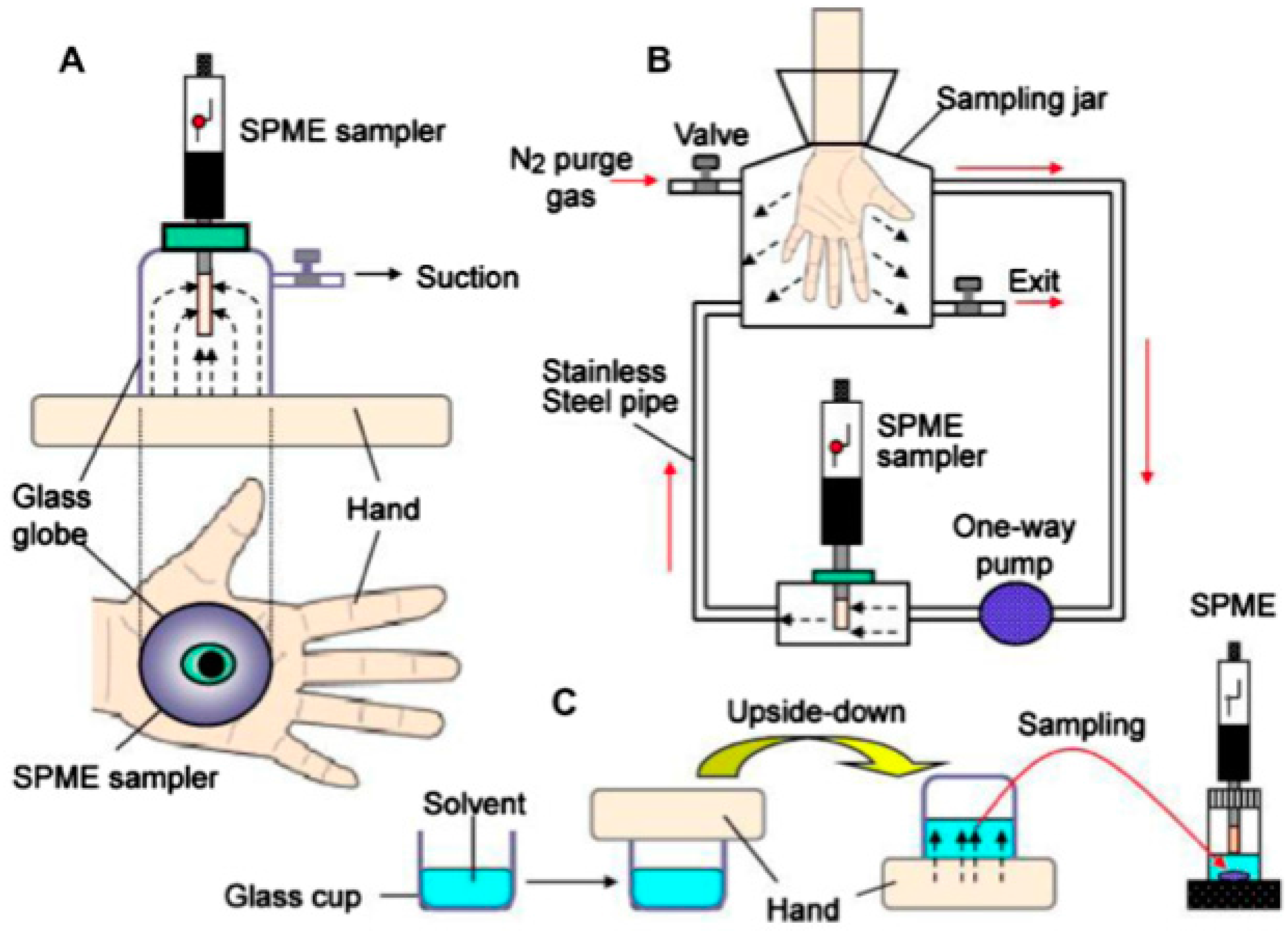
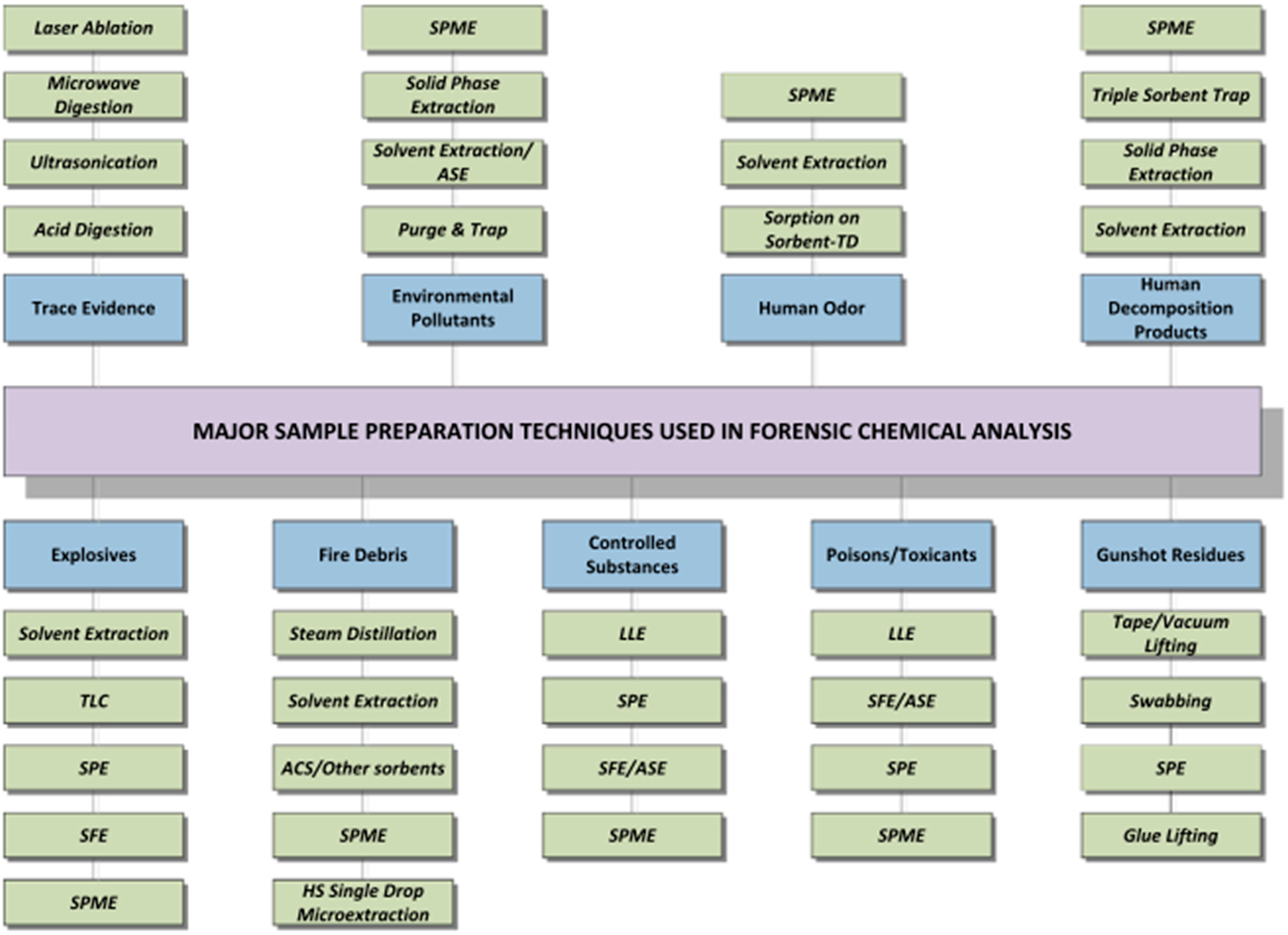

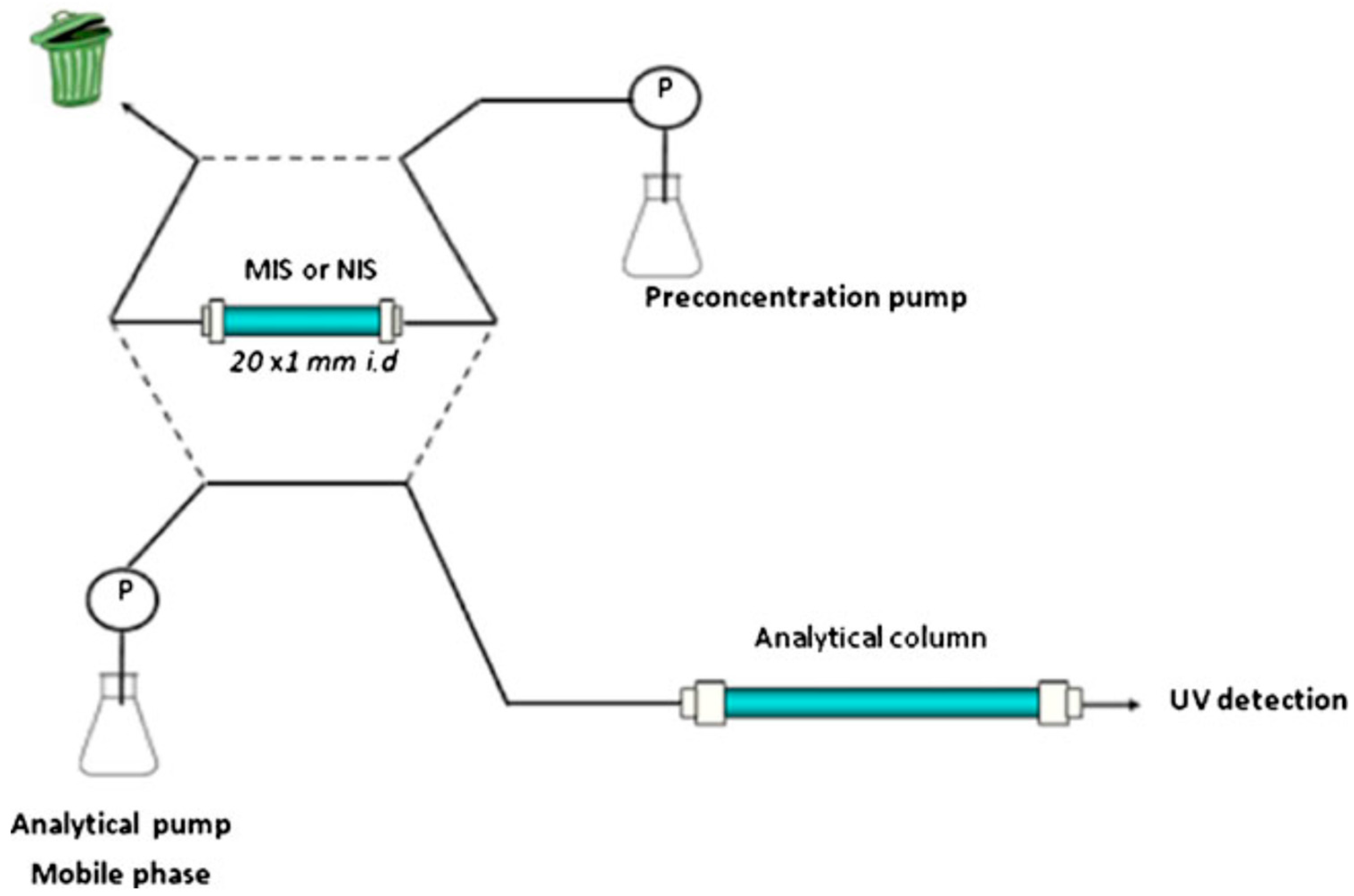
| Combined Devices | Methods | Imprinting Materials | Analyte | Media | Attributes | Ref. | |
|---|---|---|---|---|---|---|---|
| Legal/illicit drugs | LC-MS-MS | SPE | MAA and EDMA based monolith | Diazepam | Hair | LOD: 0.09 | [6,7] |
| EI-MS | SPE | MAA and EDMA based monolith | Benzodiazepines | Plasma | LOQ: <10 µg/L | [8] | |
| Electrochemical sensor | Composite NPs | PPyr sol-gel gold nanoparticles | Lorazepam | Artificial soln. | LOD: 0.09 nM | [9] | |
| GC-MS | SPE | MAA and EDMA based monolith | Δ9-THC-OH | Urine | LOD: 2.5–1 ng/mL | [10] | |
| LC-MS | SPE | EDMA and DVB based membrane | Cannabinoids | Plasma and urine | LOQ: 0.36 ng/L | [11] | |
| LC-MS-MS | SPE | AAm and EDMA based pill | Cannabinoids | Urine and OF | LOD: 0.75 ng/mL | [12] | |
| GC-FID | SPDE | MIP coated hollow stainless steel needle | MAMP, AMP, MDMA | Urine, saliva, hair | LOD: 12 ng/mL | [17] | |
| LC-MS | SPE | Ground and sieved MAA based monolith | LSD | Biological fluids | LOQ: of 0.2 pg/mL | [18] | |
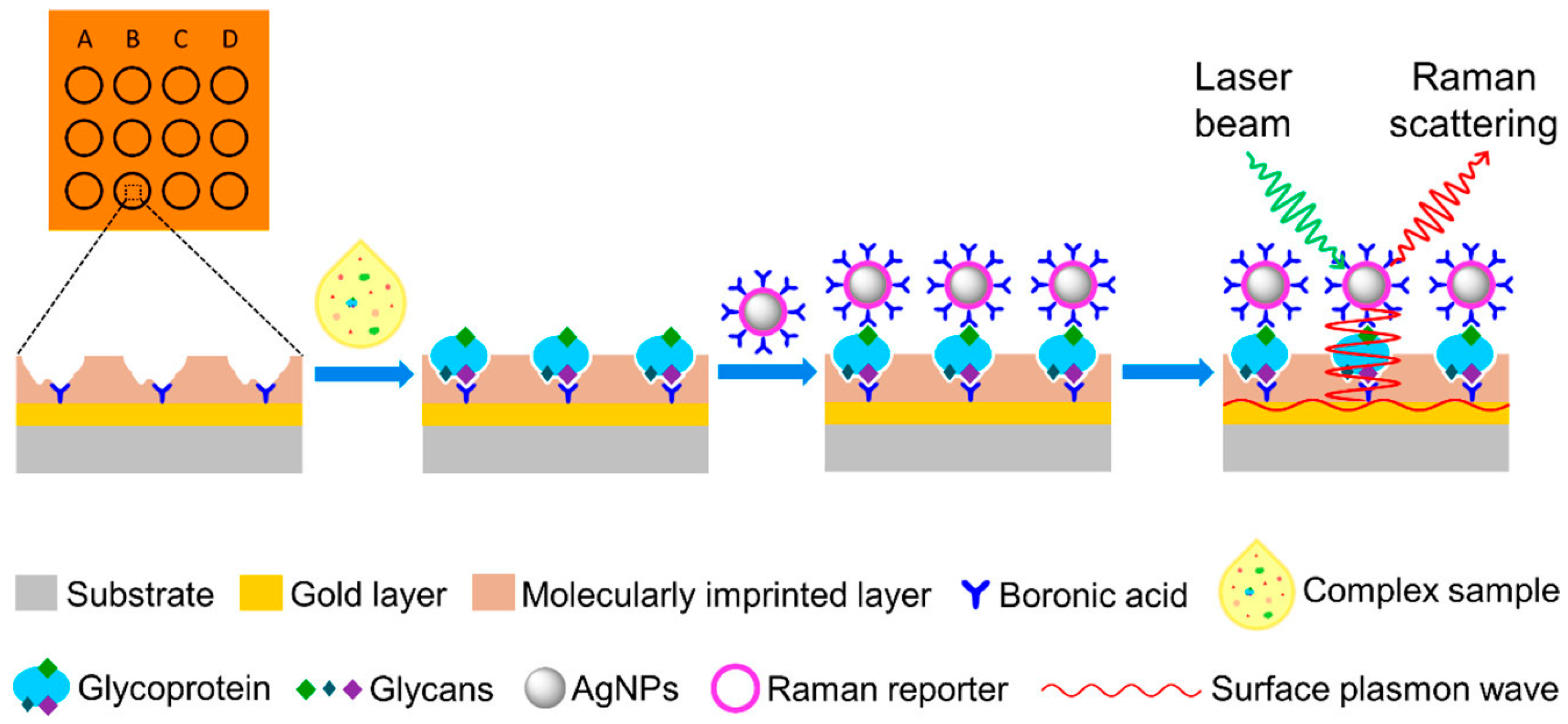
| SERS | PISA | Boronate affinity-based AuNPs | Erythropoietin | Urine | LOD: 2.9 × 10−14 M | [30] | |
| IMS | SPE | MAA and EDMA based column material | Metronidazole | Human serum | LOD: 10 μg/L | [72] | |
| Optical response without instrument | Chemosensing | Molecularly imprinted photonic hydrogels | ATR, MOR | Biological samples | LOD: 1 pg/mL (ATR) 0.1 ng/mL (MOR) | [79,81] | |
| Poisons | Quartz crystal-TSM sensor | Chemosensing | Imprinted polymer coating | Nicotine | Human serum and urine | LOD: 2.5 × 10−8 M | [20] |
| Electrochemical sensor | LSV | PPDA/SWNTs composite film | Brucine | Human serum | LOD: 2.1 × 10−7 M | [32] | |
| Electrochemical sensor | EIS | MAA and EDMA based NPs | Arsenic | Biological fluids | LOD: 5.0 × 10−7 | [33] | |
| Magnets and UV light | Magnetic response | Oleic acid modified Fe3O4 and Photoswitchable monomer | Caffeine | Water and beverage samples | Fast rebinding kinetics and high selectivity | [35] | |
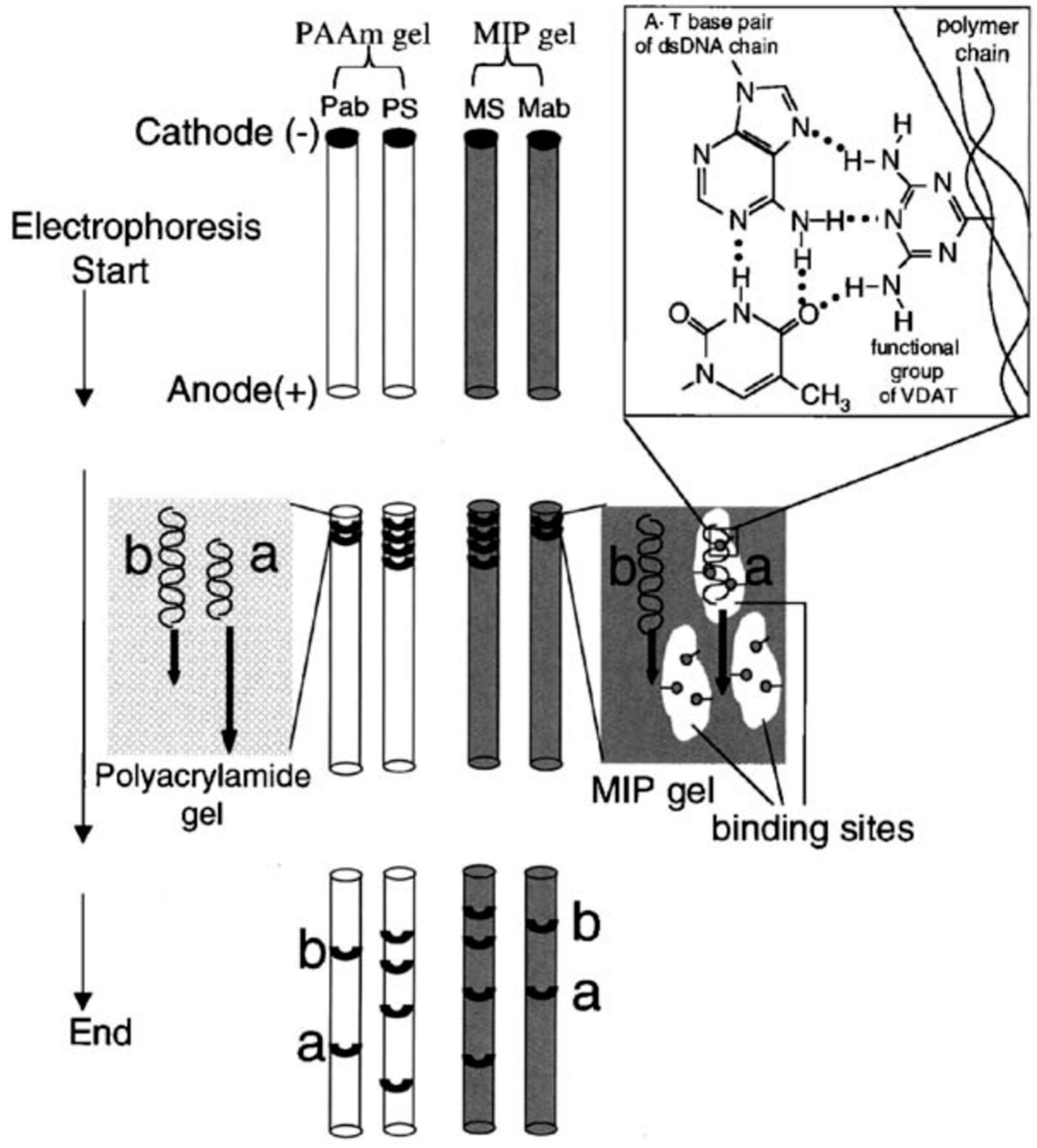
| DNA | Electrophoresis | GE | MIP gel as an electrophoretic matrix | Double strand DNA | Mixed-DNA sample | Simple and cost-effective detection | [36] |
| No instrument | Adsorption | Hydrophobic cryogels | Plasmid DNA | E. coli lysate | Q: 45.31 mg DNA/g | [39] | |
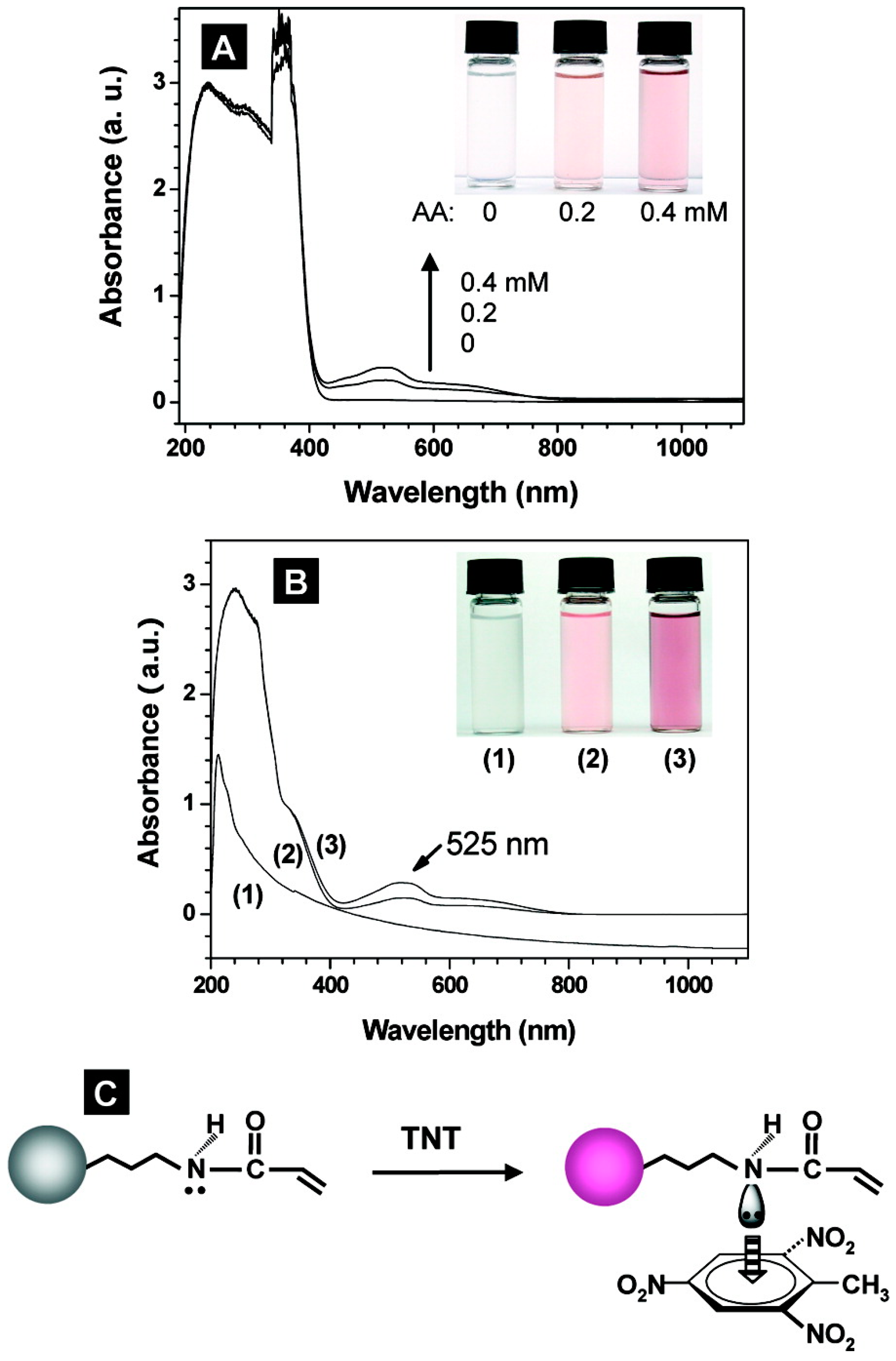
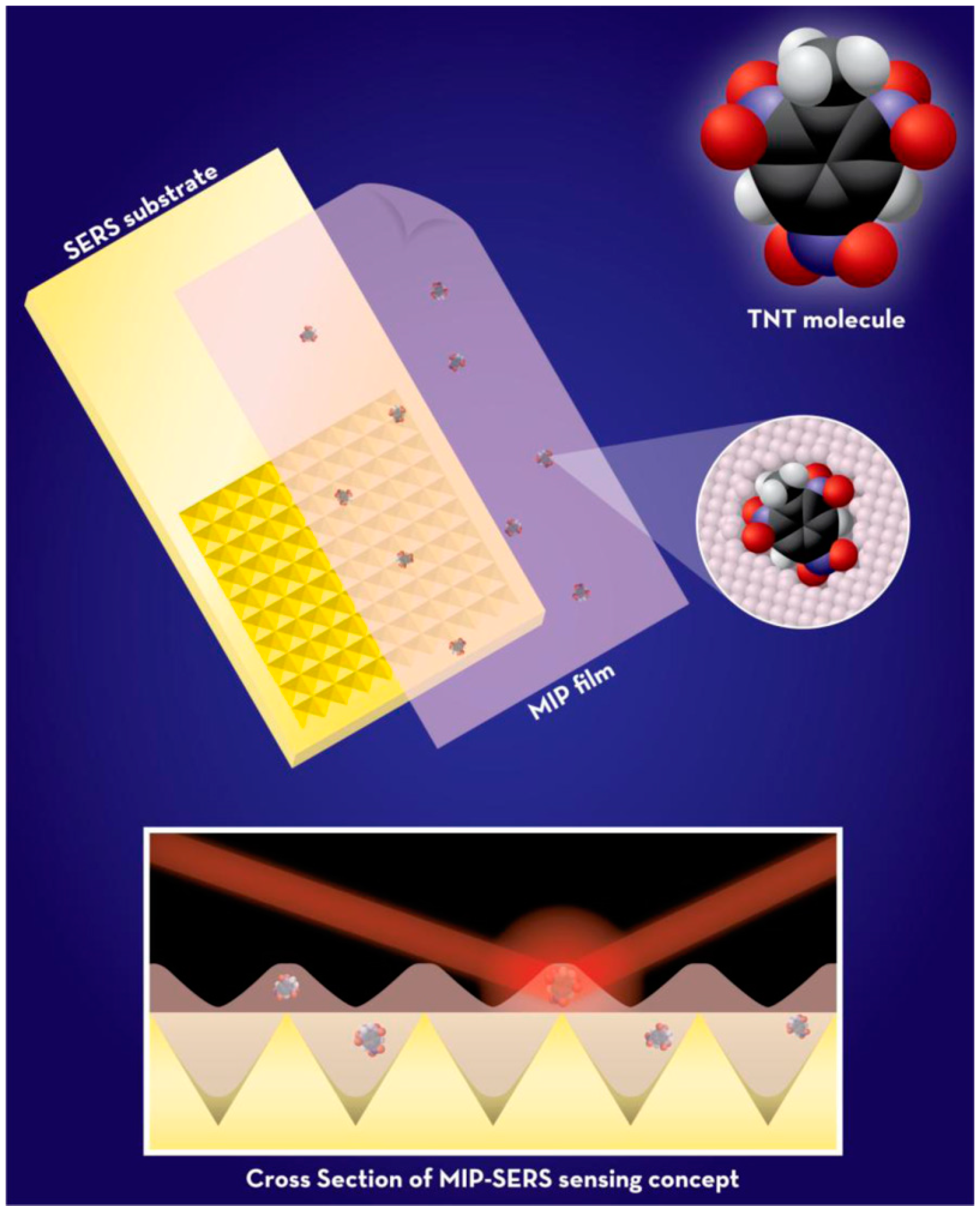
| Explosives and gunshot residues | SERS | Xerogels | Spin casted xerogel films | TNT | Artificial soln. | LOD: 3 µM | [43] |
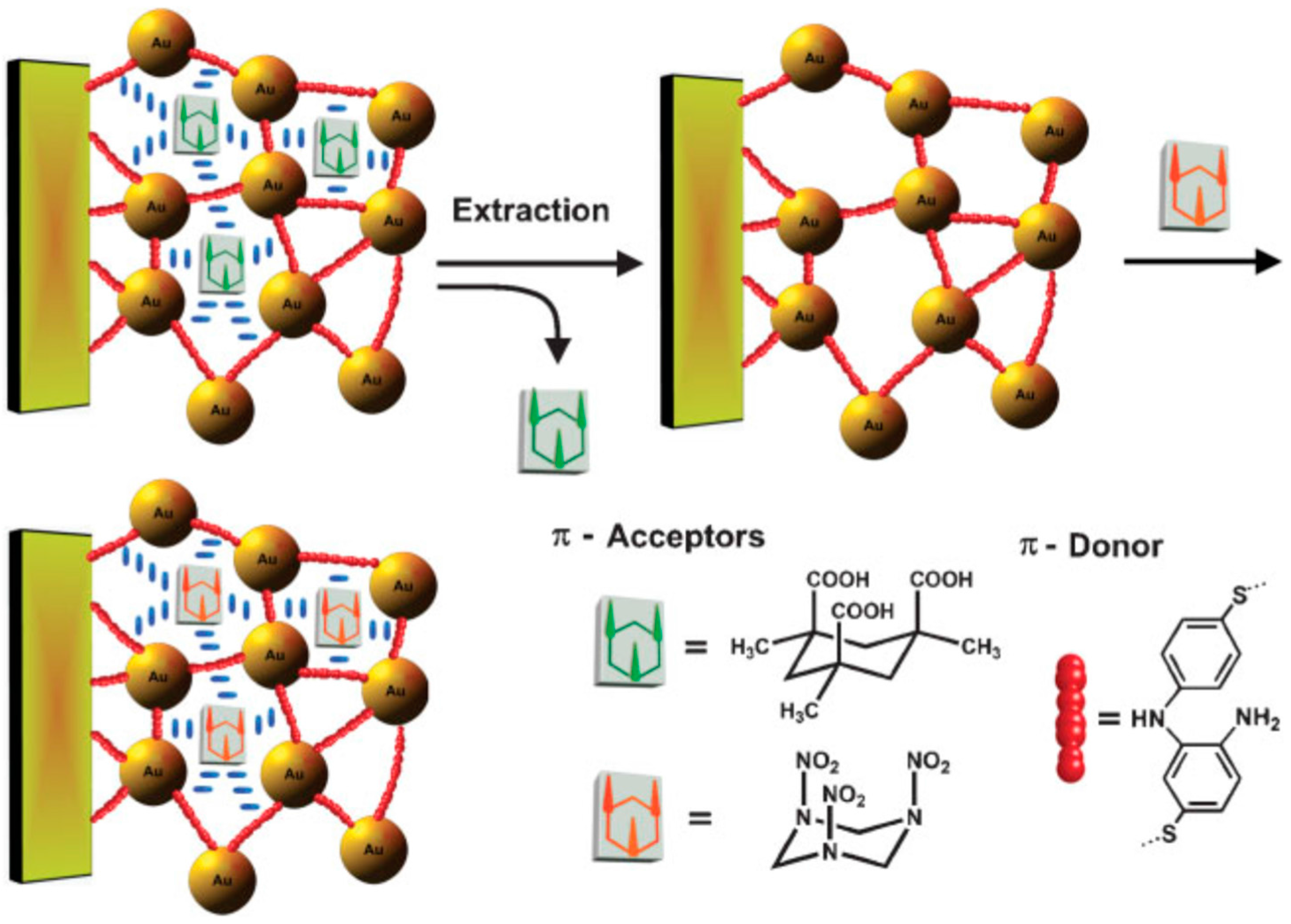
| SPR | Optical response | Au-NPs based composite | RDX, PETN, NG, EGDN | Artificial soln. | LOD: 12 fM–20 pM | [44,45] | |
| RP-LC | Online SPE | Sol-Gel organosilane | Nitroaromatic explosives | Post-blast samples | Extraction recoveries higher than 90% | [46,48] | |
| HPLC | SPE | Polymeric microparticles | Diphenylamine (DPA) | Gunshot residues | Retention up to 90% | [52] | |
| IMS | SPE | AAm and EDMA based particles | Nitroaromatics | Surface water | On-site detection | [74] | |
| Fire accelerants | Chemiresistor sensor | Chemosensing | MWCNs-PMMA composites | Ethanol vapor | Air | LOD: 0.5 ppm | [54] |
| QCM | Mass sensitive | PMMA and DVB based particles | Xylene and toluene | Air | Simple and reliable | [23] | |
| Warfare agents | Electrochemical sensor | Potensiometry | MMA, VP and EDMA based particles | MPA | Natural water | 5 × 10−8 M | [56] |
| Fluorescence spectrometer | Fluorescence detection | Molecularly imprinted silica particles | Ricin | Artificial soln. | 2–10 μM range | [85] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yılmaz, E.; Garipcan, B.; Patra, H.K.; Uzun, L. Molecular Imprinting Applications in Forensic Science. Sensors 2017, 17, 691. https://doi.org/10.3390/s17040691
Yılmaz E, Garipcan B, Patra HK, Uzun L. Molecular Imprinting Applications in Forensic Science. Sensors. 2017; 17(4):691. https://doi.org/10.3390/s17040691
Chicago/Turabian StyleYılmaz, Erkut, Bora Garipcan, Hirak K. Patra, and Lokman Uzun. 2017. "Molecular Imprinting Applications in Forensic Science" Sensors 17, no. 4: 691. https://doi.org/10.3390/s17040691






