An All-Solid-State pH Sensor Employing Fluorine-Terminated Polycrystalline Boron-Doped Diamond as a pH-Insensitive Solution-Gate Field-Effect Transistor
Abstract
:1. Introduction
2. Experimental
2.1. SGFET Fabrication
2.2. Surface Modification of Diamond for pH-Insensitive/pH-Sensitive BDD SGFET
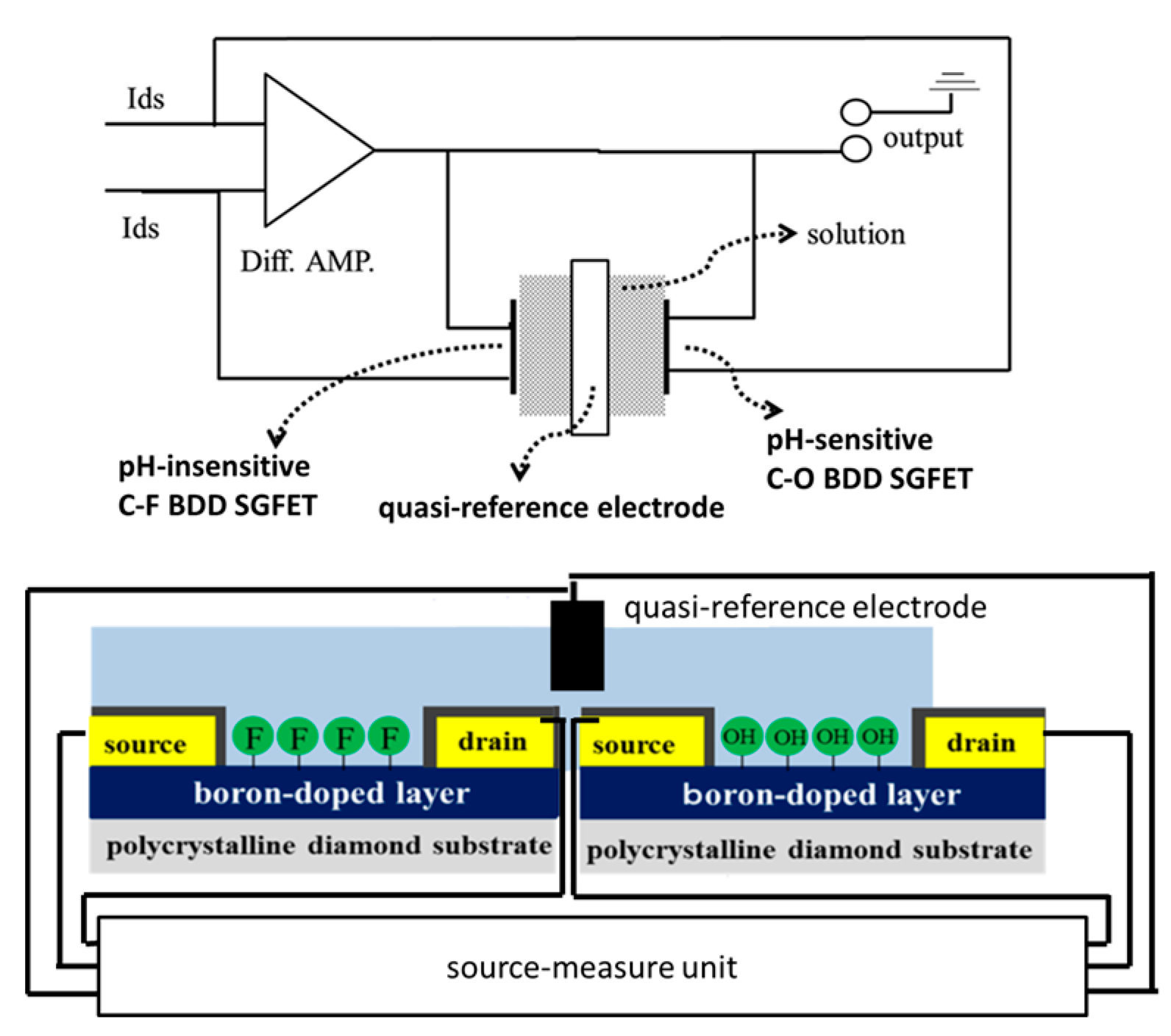
2.3. Measuring System for Characterization of FET
3. Results and Discussion
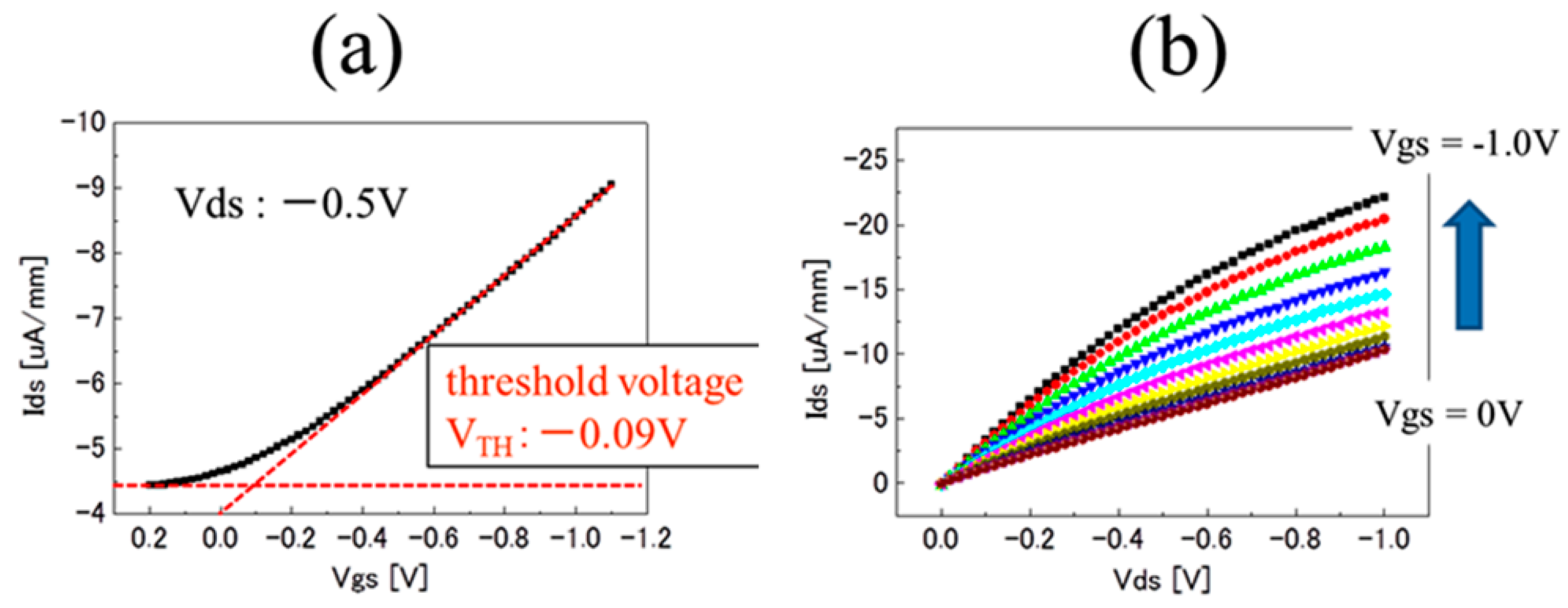
3.1. Characterization of pH-Insensitive C-F BDD SGFET
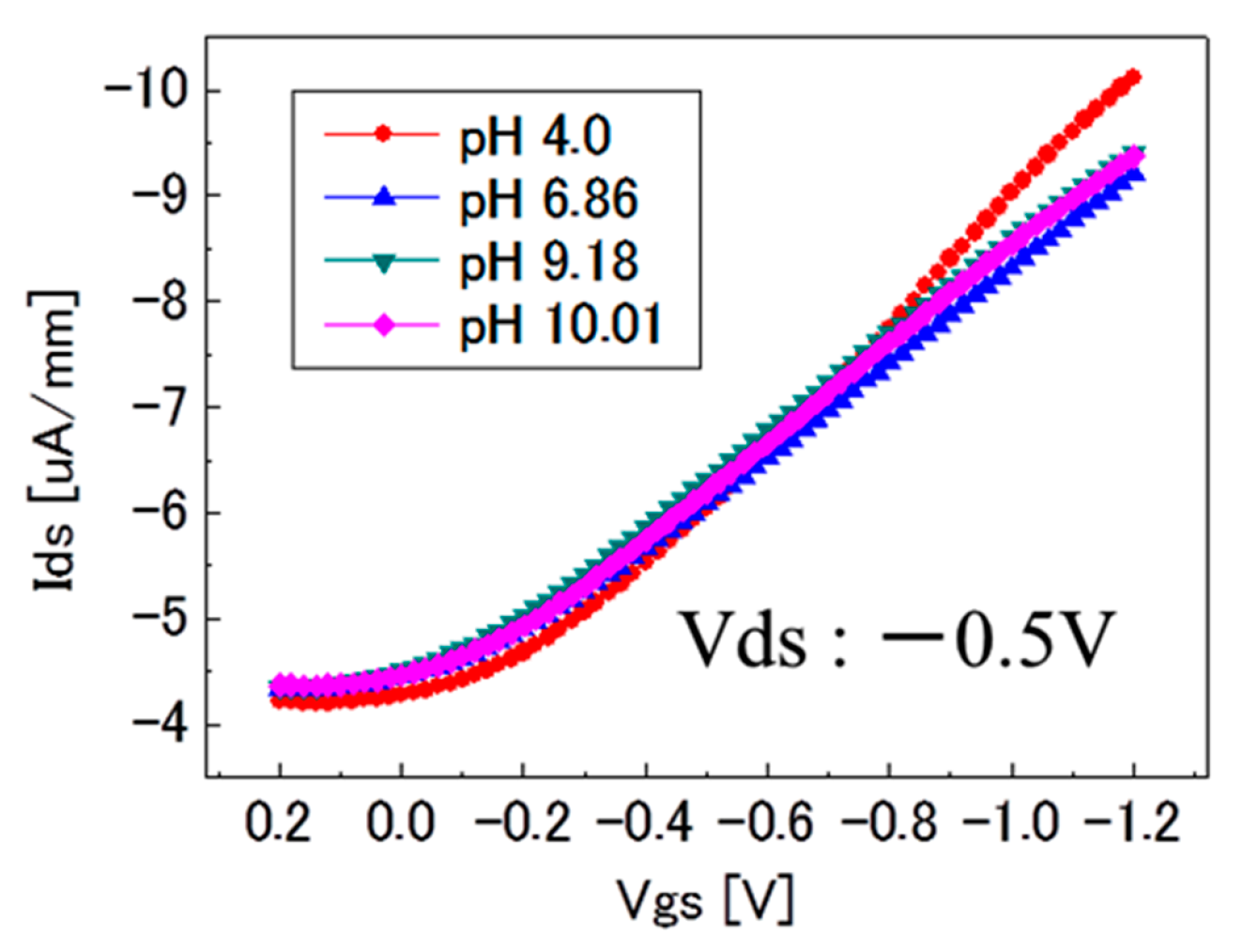
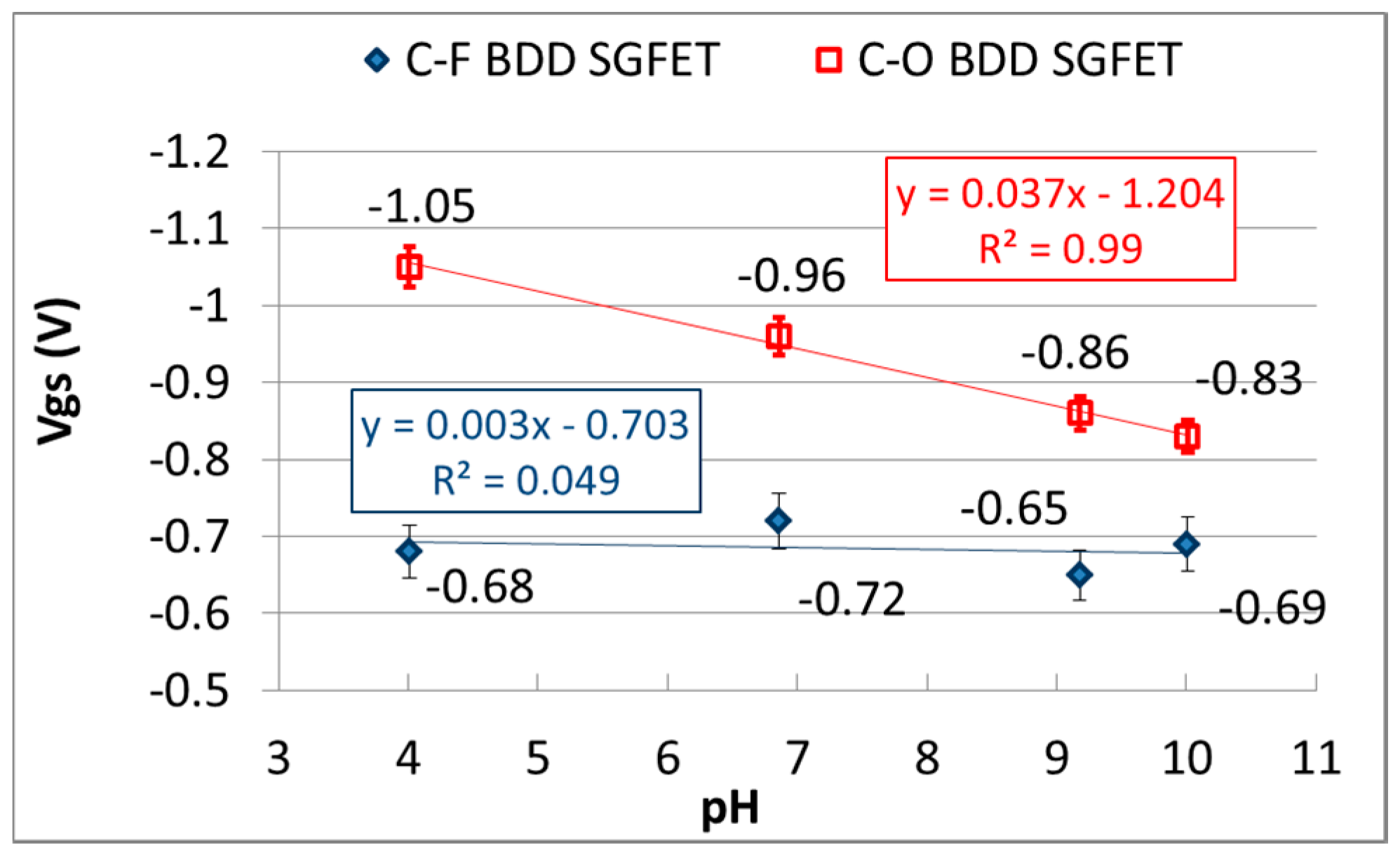
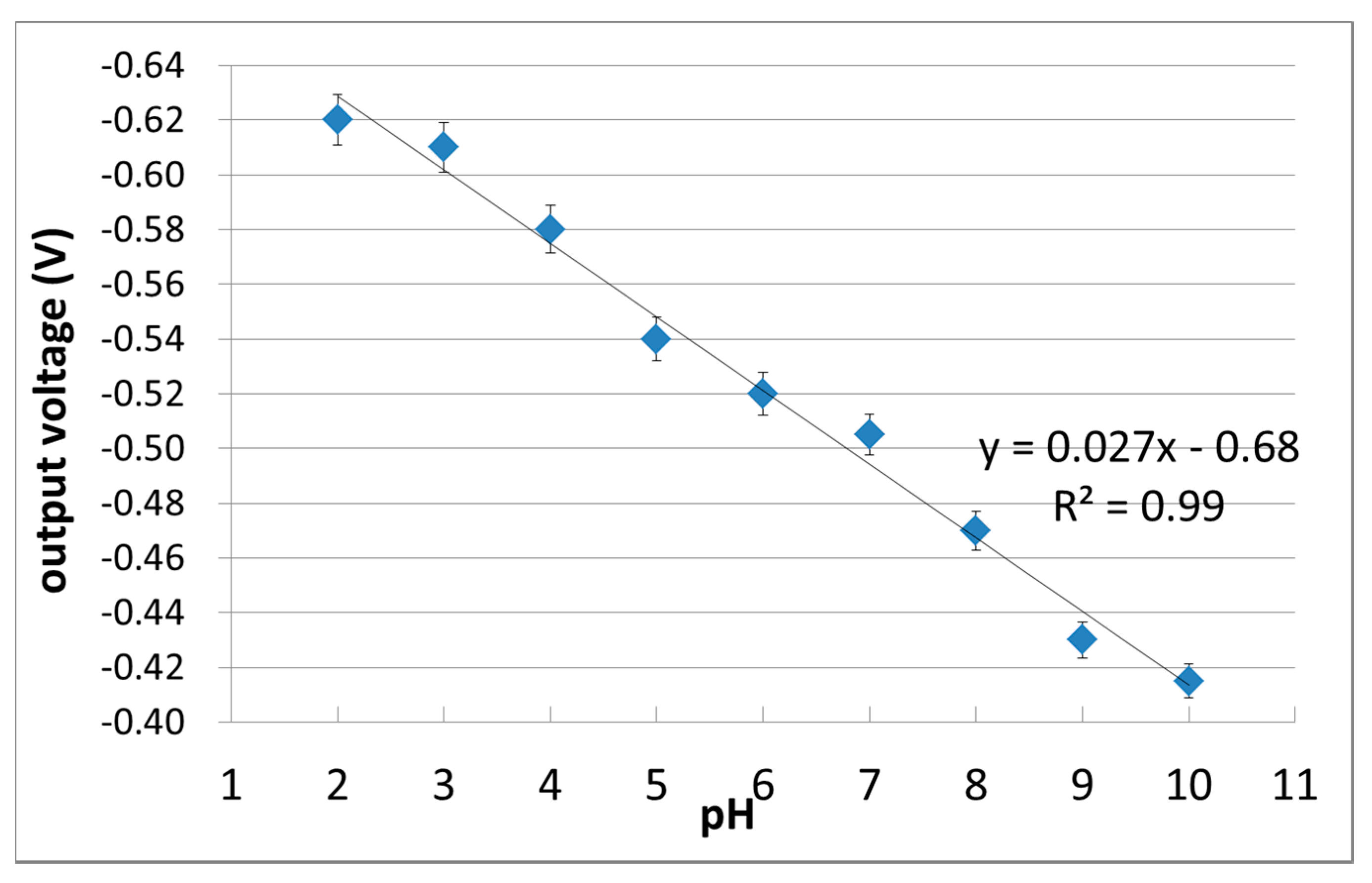
3.2. pH-Insensitivity of C-F BDD SGFET and pH-Sensitive C-O BDD SGFET
3.3. Differential FET Sensing, Using pH-Insensitive C-F BDD SGFET and pH-Sensitive C-O BDD SGFET
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ISFET | ion-sensitive field-effect transistor |
| SGFET | solution-gate field-effect transistor |
| REFET | reference field-effect transistor |
| C-O BDD | oxygen-terminated boron-doped diamond |
| C-F BDD | fluorine-terminated boron-doped diamond |
| QRE | quasi-reference electrode |
| BDD | boron-doped diamond |
| Ids | drain-source current |
| Vgs | gate-source voltage |
| Vds | drain-source voltage |
| PVC | poly (vinyl chloride) |
| ICP | inductively coupled plasma |
| CVD | chemical vapor deposition |
References
- Bergveld, P. Development of an ion-sensitive solid-state device for neurophysiological measurements. IEEE Trans. Biomed. Eng. 1970, 17, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.L.; Chou, J.C.; Chen, Y.C. Study of the pH-ISFET and EnFET for Biosensor Applications. J. Med. Biol. Eng. 2001, 21, 135–146. [Google Scholar]
- Voigt, H.; Schitthelm, F.; Lange, T.; Kullick, T.; Ferretti, R. Diamond-like carbon-gate pH-ISFET. Sens. Actuators B Chem. 1997, 44, 441–445. [Google Scholar] [CrossRef]
- Kawarada, H.; Araki, Y.; Sakai, T.; Ogawa, T.; Umezawa, H. Electrolyte-solution- gate FETs using diamond surface for biocompatible ion sensors. Phys. Status Solidi (a) 2001, 185, 79–83. [Google Scholar] [CrossRef]
- Edgington, R.; Ruslinda, A.R.; Sato, S.; Ishiyama, Y.; Tsuge, K.; Ono, T.; Kawarada, H.; Jackman, R.B. Boron δ-doped (111) diamond solution gate field-effect transistors. Biosens. Bioelectron. 2012, 33, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Edgington, R.; Sato, S.; Ishiyama, Y.; Morris, R.; Jackman, R.B.; Kawarada, H. Growth and electrical characterisation of δ-doped boron layers on (111) diamond surfaces. J. Appl. Phys. 2012, 111, 033710. [Google Scholar] [CrossRef]
- Sasaki, Y.; Kawarada, H. Low drift and small hysteresis characteristics of diamond electrolyte-solution-gate FET. J. Phys. D Appl. Phys. 2010, 43, 374020. [Google Scholar] [CrossRef]
- Shintani, Y.; Ibori, S.; Igarashi, K.; Naramura, T.; Inaba, M.; Kawarada, H. Polycrystalline boron-doped diamond with an oxygen-terminated surface channel as an electrolyte-solution-gate field-effect transistor for pH sensing. Electrochim. Acta 2016, 212, 10–15. [Google Scholar] [CrossRef]
- Shintani, Y.; Ibori, S.; Kawarada, H. Polycrystalline Boron-doped Diamond Solution-gate Field-effect Transistor with a Carboxyl-terminated Channel for DNA. J. Appl. Phys. 2017. submitted. [Google Scholar]
- Naramura, T.; Inaba, M.; Mizuno, S.; Igarashi, K.; Kida, E.; Falina, S.; Shintani, Y.; Kawarada, H. Threshold voltage control of electrolyte solution gate field-effect transistor by electrochemical oxidation. Appl. Phys. Lett. 2017. submitted. [Google Scholar]
- Shintani, Y.; Kawarada, H. Polycrystalline Boron-doped diamond electrolyte-solution-gate field-effect transistor for an application to the measurement of water percentage in ethanol. Anal. Sci. 2017. submitted. [Google Scholar]
- Errachid, A.; Bausells, J.; Jaffrezic-Renault, N. A simple REFET for pH detection in differential mode. Sens. Actuators B Chem. 1999, 60, 43–48. [Google Scholar] [CrossRef]
- Smith, R.L.; Scott, D.C. An integrated sensor for electrochemical measurements. IEEE Trans. Biomed. Eng. 1986, 33, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.; Chang, C.; Chao, K.; Chen, J. Development of FET-type Reference Electrodes for pH-ISFET Applications. J. Electrochem. Soc. 2010, 157, J143–J148. [Google Scholar] [CrossRef]
- Salm, E.; Zhong, Y.; Reddy, B.; Guevara, C.; Swaminathan, V.; Liu, Y.; Bashir, R. Electrical Detection of Nucleic Acid Amplification Using an On-chip Quasi-reference electrode and a PVC REFET. Anal. Chem. 2014, 86, 6968–6975. [Google Scholar] [CrossRef] [PubMed]
- Ruslinda, A.R.; Ishiyama, Y.; Penmatsa, V.; Ibori, S.; Kawarada, H. Repulsive effects of hydrophobic diamond thin films on biomolecule detection. Appl. Surf. Sci. 2014, 328, 314–318. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shintani, Y.; Kobayashi, M.; Kawarada, H. An All-Solid-State pH Sensor Employing Fluorine-Terminated Polycrystalline Boron-Doped Diamond as a pH-Insensitive Solution-Gate Field-Effect Transistor. Sensors 2017, 17, 1040. https://doi.org/10.3390/s17051040
Shintani Y, Kobayashi M, Kawarada H. An All-Solid-State pH Sensor Employing Fluorine-Terminated Polycrystalline Boron-Doped Diamond as a pH-Insensitive Solution-Gate Field-Effect Transistor. Sensors. 2017; 17(5):1040. https://doi.org/10.3390/s17051040
Chicago/Turabian StyleShintani, Yukihiro, Mikinori Kobayashi, and Hiroshi Kawarada. 2017. "An All-Solid-State pH Sensor Employing Fluorine-Terminated Polycrystalline Boron-Doped Diamond as a pH-Insensitive Solution-Gate Field-Effect Transistor" Sensors 17, no. 5: 1040. https://doi.org/10.3390/s17051040




