A Simple, Cost-Effective Sensor for Detecting Lead Ions in Water Using Under-Potential Deposited Bismuth Sub-Layer with Differential Pulse Voltammetry (DPV)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Apparatus and Reagents
2.2. Design and Fabrication of the Sensor
2.3. Modification and Preparation of the Lead Ions Sensor
2.3.1. Chemical Pretreatment of the Thin Gold Film Electrode Elements
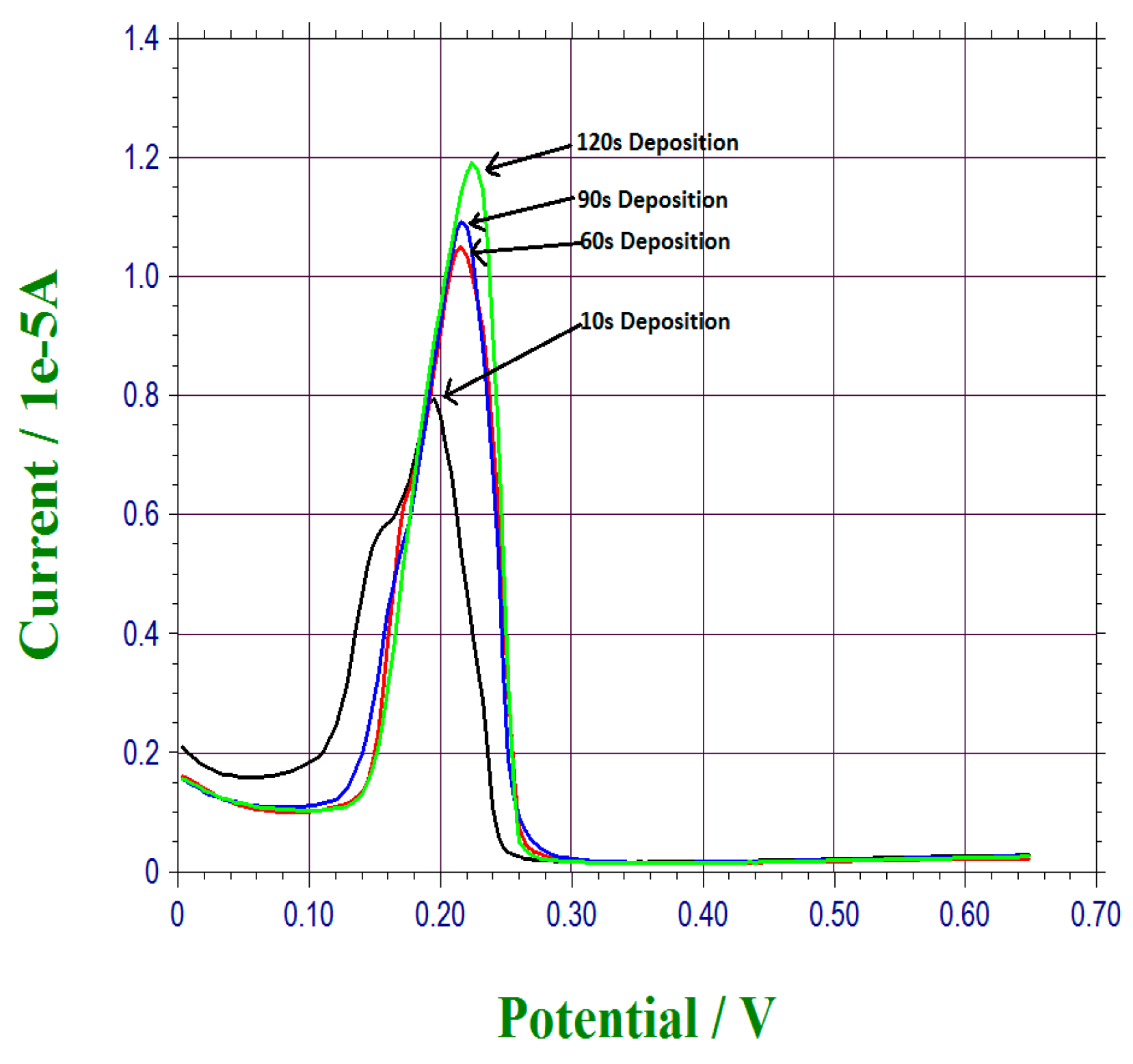
2.3.2. Under-Potential Deposition of Bismuth on the Thin Gold Film Based Sensor
2.3.3. Surface Characterization of Bismuth Sublayer with XPS and Tof-SIMS
3. Results and Discussion
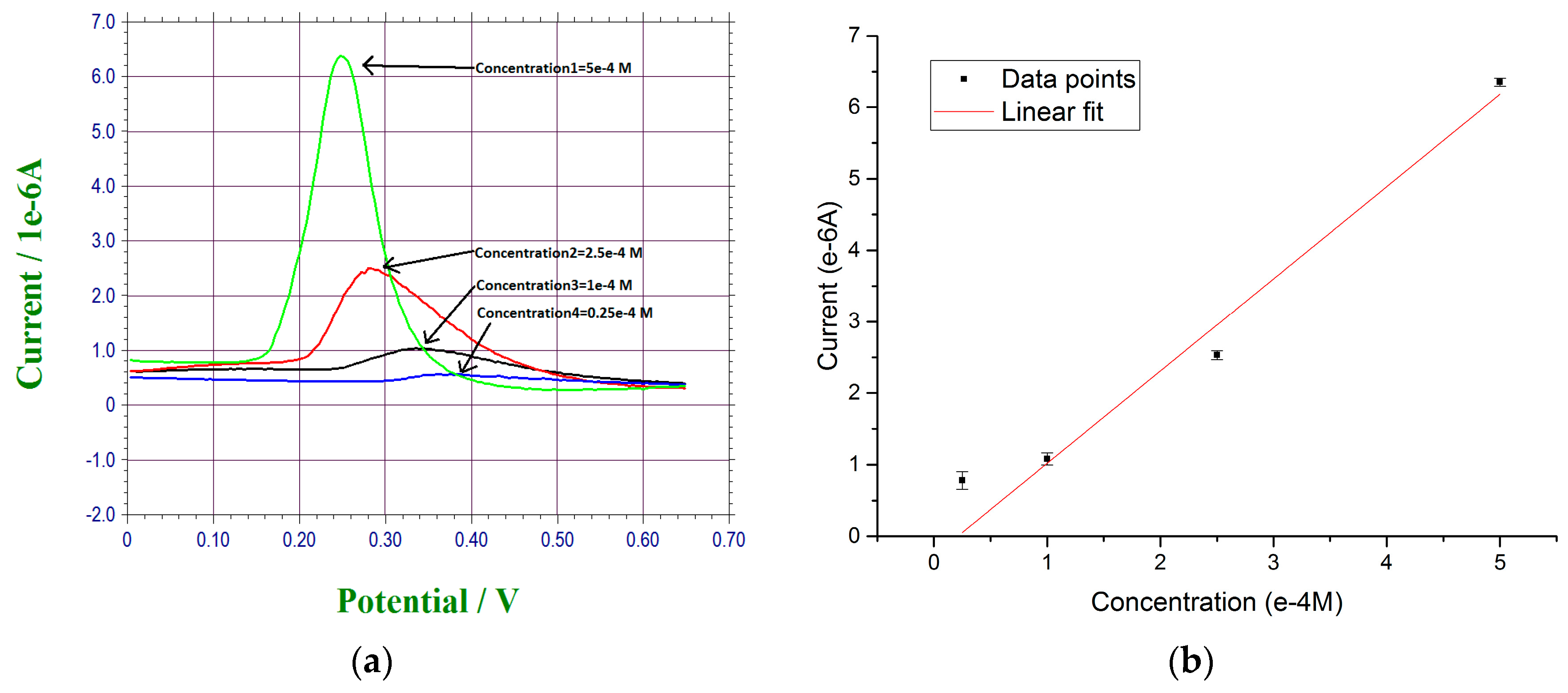
3.1. Anodic Stripping of Lead Ions by Differential Pulse Voltammetry (DPV)
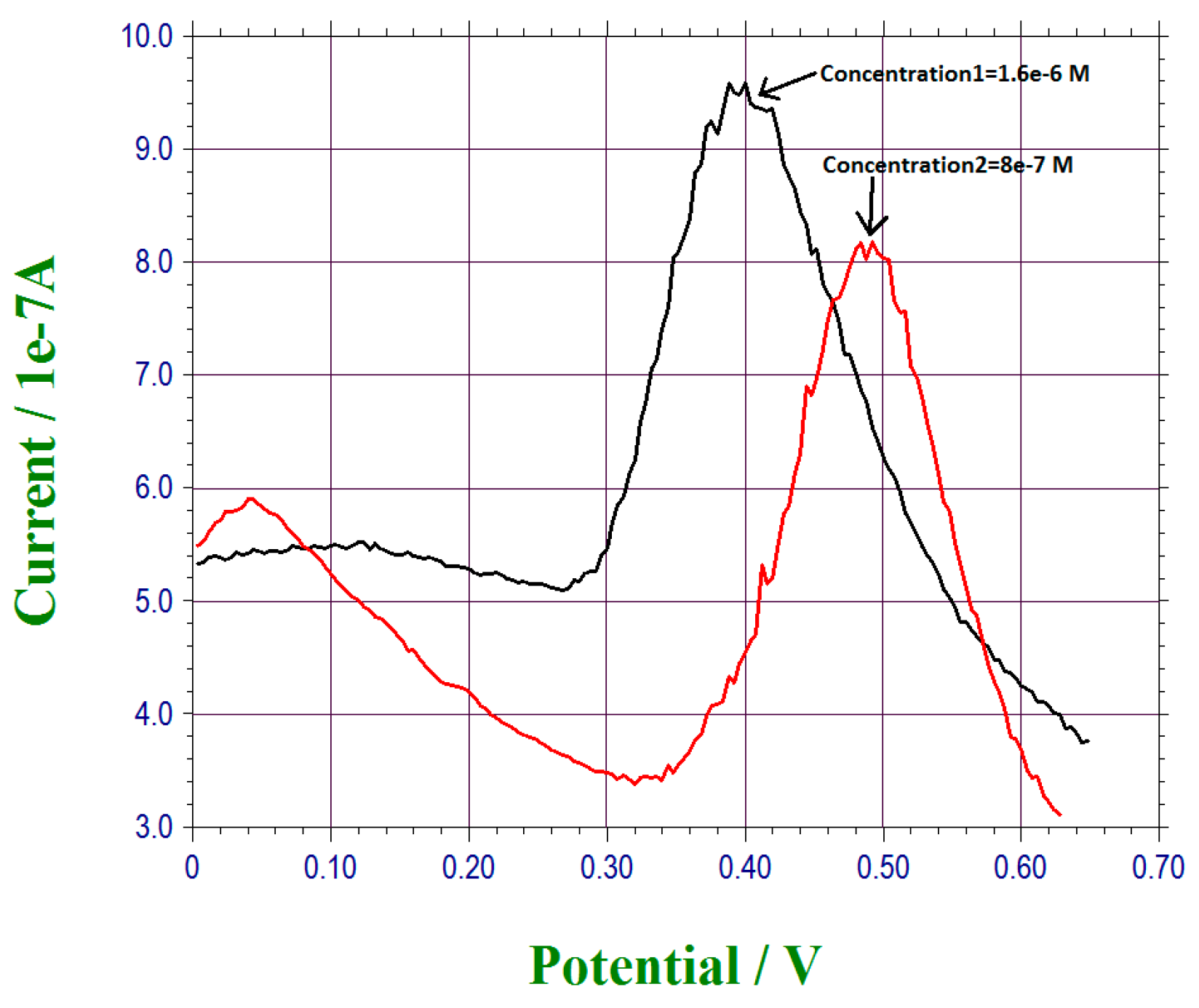
3.2. Detection of Lead Ions in Tap Water
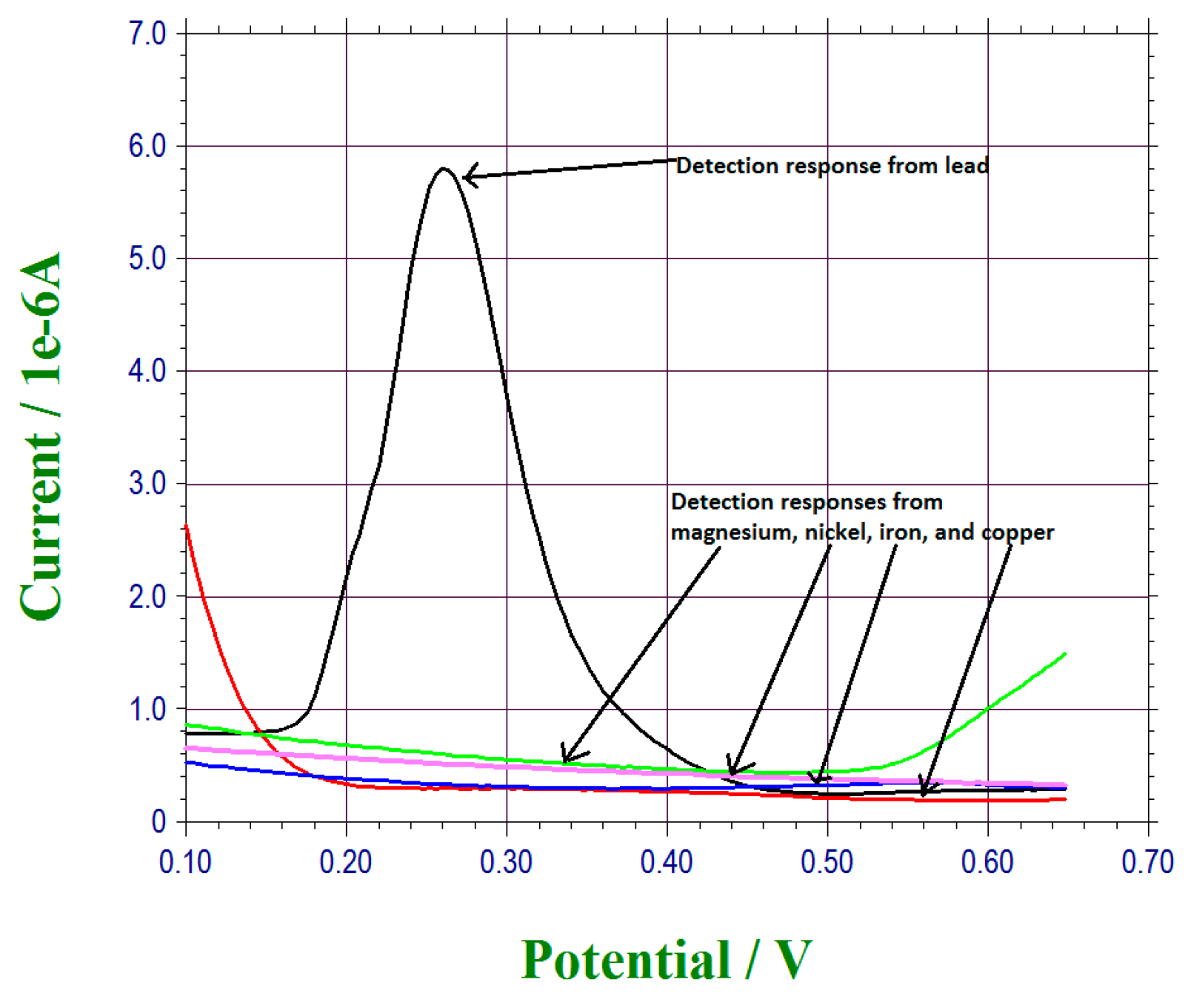
3.3. Interference Study of This Lead Ions Sensor
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Satcher, D. Screening Young Children for Lead Poisoning: Guidance for State and Local Public Health Officials; Center for Disease Control and Prevention: Atlanta, GA, USA, 1997. [Google Scholar]
- Center for Disease Control and Prevention. Blood Lead Levels in Residents of Homes with Elevated Lead in Tap Water—District of Columbia, 2004; Center for Disease Control and Prevention: Washington, DC, USA, 2004; Volume 53, pp. 268–270. [Google Scholar]
- National Center for Environmental Health, Division of Emergency and Environmental Health Services. Sources of Lead-Water. 2016. Available online: https://www.cdc.gov/nceh/lead/tips/water.htm (accessed on 25 April 2017).
- National Center for Environmental Health, Division of Emergency and Environmental Health Services Childhood. Lead Poisoning Data, Statistics, and Surveillance. 2016. Available online: https://www.cdc.gov/nceh/lead/data/index.htm (accessed on 19 July 2016).
- Wanekaya, A.; Sadik, O. Electrochemical detection of lead using overoxidized polypyrrole films. J. Electroanal. Chem. 2002, 537, 135–143. [Google Scholar] [CrossRef]
- Arduini, F.; Calvo, J.; Amine, A.; Palleschi, G. Bismuth-modified electrodes for lead detection. Trends Anal. Chem. 2010, 29, 1295–1304. [Google Scholar] [CrossRef]
- Maria das Graças, A.K.; de Andrade, J.B.; de Jesus, D.S.; Lemos, V.A.; Bandeira, M.L.; dos Santos, W.N.; Bezerra, M.A.; Amorim, F.A.; Souza, A.S.; Ferreira, S.L. Separation and preconcentration procedures for the determination of lead using spectrometric techniques: A review. Talanta 2006, 69, 16–24. [Google Scholar]
- Welz, B.; Sperling, M. Atomic Absorption Spectrometry, 3rd ed.; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]
- Kanyong, P.; Rawlinson, S.; Davis, J. Gold nanoparticle modified screen-printed carbon arrays for the simultaneous electrochemical analysis of lead and copper in tap water. Microchim. Acta 2016, 183, 2361–2368. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2001. [Google Scholar]
- Bockris, J.O.M.; Reddy, A.K.N.; Gambos-Aldeco, M.E. Modern Electrochemistry 2A; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2000. [Google Scholar]
- Molazemhosseini, A.; Magagnin, L.; Vena, P.; Liu, C.-C. Single-use disposable electrochemical label-free immunosensor for detection of glycated hemoglobin (HbA1c) using differential pulse voltammetry (DPV). Sensors 2016, 16, 1024. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Molazemhosseini, A.; Liu, C.C. A single-use, in vitro biosensor for the detection of T-tau protein, a biomarker of neuro-degenerative disorders, in PBS and human serum using differential pulse voltammetry (DPV). Biosensors 2017, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Willner, I.; Riklin, A. Electrical communication between electrode and NAD(P) + -dependent enzymes using pyrroloquiuolinequinone-electrodes in a self-assembled monolayer configuration: Design of a new class of amperometric biosensors. Anal. Chem. 1994, 66, 1535–1539. [Google Scholar] [CrossRef]
- Campuzano, S.; Glavez, R.; Dedrero, M.; DeVillena, F.J.M.; Pingarron, J.M. Preparation characterization and application of alkanethiol self-assembled monolayers modified with tetrathiafulvalene and glucose oxidase at a gold disk electrode. J. Electroanal. Chem. 2002, 526, 92–100. [Google Scholar] [CrossRef]
- Chow, E.; Hibbert, D.B.; Gooding, J.J. Electrochemical detection of lead ions via the covalent attachment of human angiotensin I to mercaptopropionic acid and thioctic acid self-assembled monolayers. Anal. Chim. Acta 2005, 543, 167–176. [Google Scholar] [CrossRef]
- Salih, F.; Ouarzane, A.; Rhazi, M. Electrochemical detection of lead(II) at bismuth/poly(1,8-diaminonaphthalene) modified carbon paste electrode. Arabian J. Chem. 2015. [Google Scholar] [CrossRef]
- Wang, J.; Lu, J.; Hocevar, S.B.; Farias, P.A.M. Bismuth-coated electrodes for anodic stripping voltammetry. Anal. Chem. 2000, 72, 3218–3222. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, J.; Hocevar, S.B.; Ogorevc, B. Bismuth-coated screen-printed electrodes for stripping voltammetric measurements of trace lead. Electroanalysis 2001, 13, 13–16. [Google Scholar] [CrossRef]
- Romann, T.; Kallip, S.; Sammelselg, V.; Lust, E. Bismuth microelectrode system with in situ renewable surface for electroanalysis and adsorption studies. Electrochem. Commun. 2008, 10, 1008–1011. [Google Scholar] [CrossRef]
- Salles, M.O.; de Souza, A.P.R.; Naozuka, J.; de Oliveira, P.V.; Bertotti, M. Bismuth modified microelectrode for Pb(II) determination in wine using alkaline medium. Electroanalysis 2009, 21, 1439–1442. [Google Scholar] [CrossRef]
- Adzic, R.R.; Tripkovic, A.V.; Markovic, N.M. Oxygen reduction on electrode surfaces modified by foreign metal ad-atoms: Lead ad-atoms on gold. J. Electroanal. Chem. 1980, 114, 37–51. [Google Scholar] [CrossRef]
- Tamura, K.; Ocko, B.M.; Wang, J.X.; Adzic, R.R. Structure of active adlayers on bimetallic surfaces: Oxygen reduction non Au(111)with Bi adlayers. J. Phys. Chem. B 2002, 106, 3896–3901. [Google Scholar] [CrossRef]
- Oh, I.; Biggin, M.E.; Gewirth, A.A. Poisoning the active site of electrochemical reduction of dioxygen on metal monolayer modified electrode surfaces. Langmuir 2000, 16, 1397–1406. [Google Scholar] [CrossRef]
- Hubbard, A.T. Electrochemistry at well-characterized surface. Chem. Rev. 1988, 88, 633–656. [Google Scholar] [CrossRef]
- Kokkinidis, G. Underpotential deposition and electrocatalysis. J. Electroanal. Chem. Interfacial Electrochem. 1986, 201, 217–236. [Google Scholar] [CrossRef]
- Hara, M.; Nagahara, Y.; Inukai, J.; Yoshimoto, S.; Itaya, K. In situ STM study of underpotential deposition of bismuth on Au(110) in perchloric acid solution. Electrochim. Acta 2006, 51, 2327–2332. [Google Scholar] [CrossRef]
- Shao, M.J.; Xing, X.K.; Liu, C.C. Amperometric determination of carbon dioxide using a gold electrode modified by the under-potential-deposited cadmium. Electroanalysis 1990, 2, 367–372. [Google Scholar] [CrossRef]
- Shao, M.J.; Xing, X.K.; Liu, C.C. Cyclic voltammetric study of glucose oxidation on a oxide-covered platinum electrode in the presence of underpotential–deposited thallium layer. Bioelectrochem. Bioenerg. 1987, 17, 59–70. [Google Scholar] [CrossRef]
- Chen, C.H.; Kepler, K.D.; Gewirth, A.A.; Ocko, B.M.; Wang, J. Electrodeposited bismonolayers on gold(111) electrodes: Comparison of surface X-ray scattering, scanning tunneling microscopy, and atomic force microscopy lattice structures. J. Phys. Chem. 1993, 97, 7290–7294. [Google Scholar] [CrossRef]
- Solomun, T.; Kautek, W. Electrodeposition of bismuth and silver phases in nanometer-sized zero-dimensional STM-formed cavities on gold(111). Electrochim. Acta 2001, 47, 679–687. [Google Scholar] [CrossRef]
- Chebil, S.; Hafaiedh, I.; Sauriat-dorizon, H.; Jaffrezic-Resnault, N.; Errachid, A.; Ali, Z.; Korr-Youssoufi, H. Electrochemical detection of D-dimer as deep vein thrombosis marker using single-chain D-dimer antibody immobilized on functionalized polypyrrole. Biosens. Bioelectron. 2010, 26, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Hoberman, J.M. The histroy of synthetic testosterone. Sci. Am. 1995, 272, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Gumpu, M.B.; Sethuraman, S.; Krishnan, U.M.; Bosco, J.; Rayappan, B. A review on detection of heavy metal ions in water—An electrochemical approach. Sens. Actuators B Chem. 2015, 213, 515–533. [Google Scholar] [CrossRef]
- Bagal-Kestwal, D.; Karve, M.S.; Kakade, B.; Pillai, V.K. Invertase inhibition based electrochemical sensor for the detection of heavy metal ions in aqueous system: Application of ultra-microelectrode to enhance sucrose biosensor’s sensitivity. Biosens. Bioelectron. 2008, 24, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Bihari, V.; Agarwal, S.K.; Verma, V.; Kesavachandran, C.N.; Pangtey, B.S. Groundwater contaminated with hexavalent chromium [Cr(VI)]: A health survey and clinical examination of community Inhabi-Tants (Kanpur, India). PLoS ONE 2012, 7, e47877. [Google Scholar] [CrossRef] [PubMed]
- Jena, B.K.; Raj, C.R. Gold microelectrode ensembles for the simultaneous electrochemical detection of ultratrace arsenic, mercury, and copper. Anal. Chem. 2008, 80, 4836–4844. [Google Scholar] [CrossRef] [PubMed]
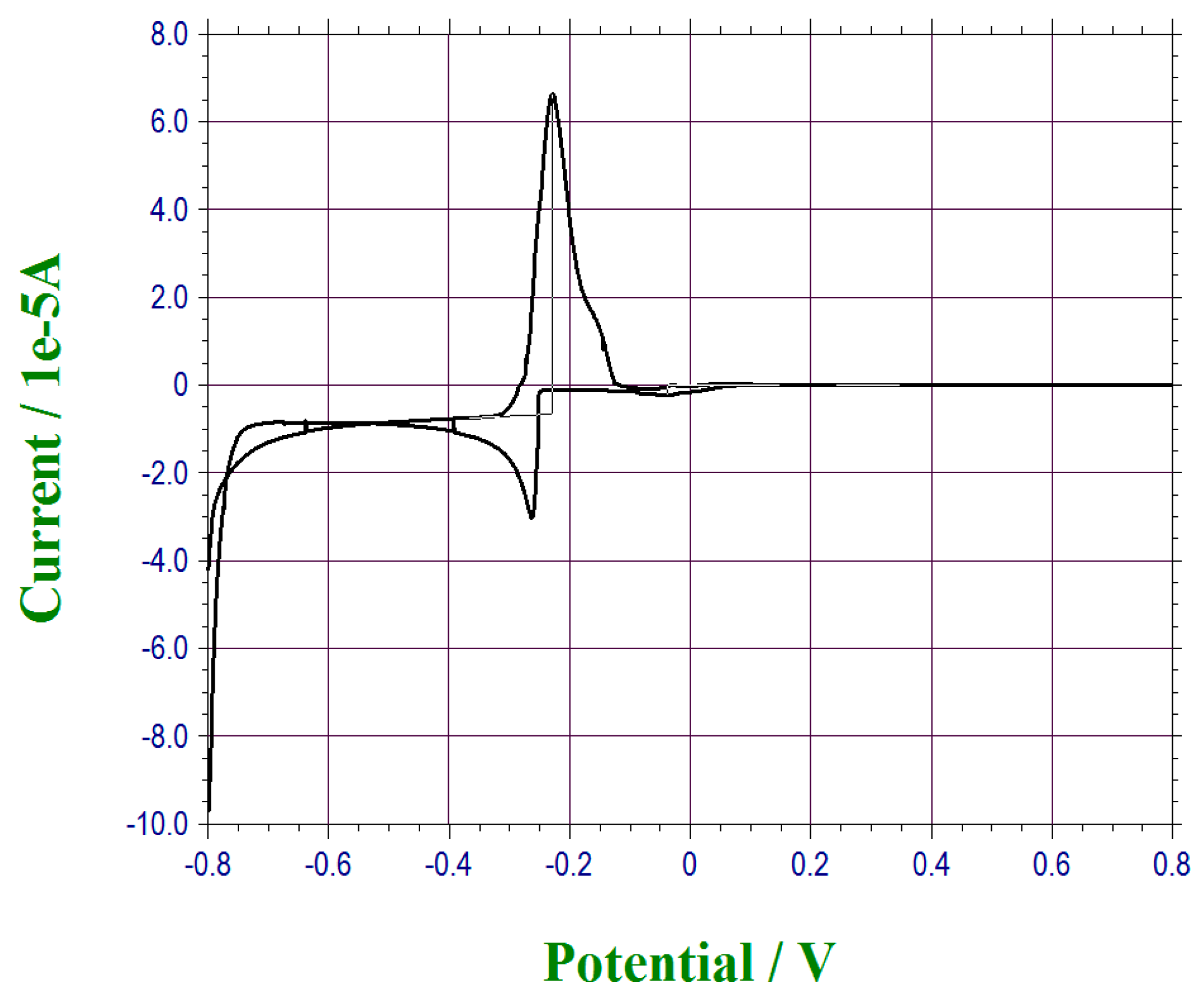
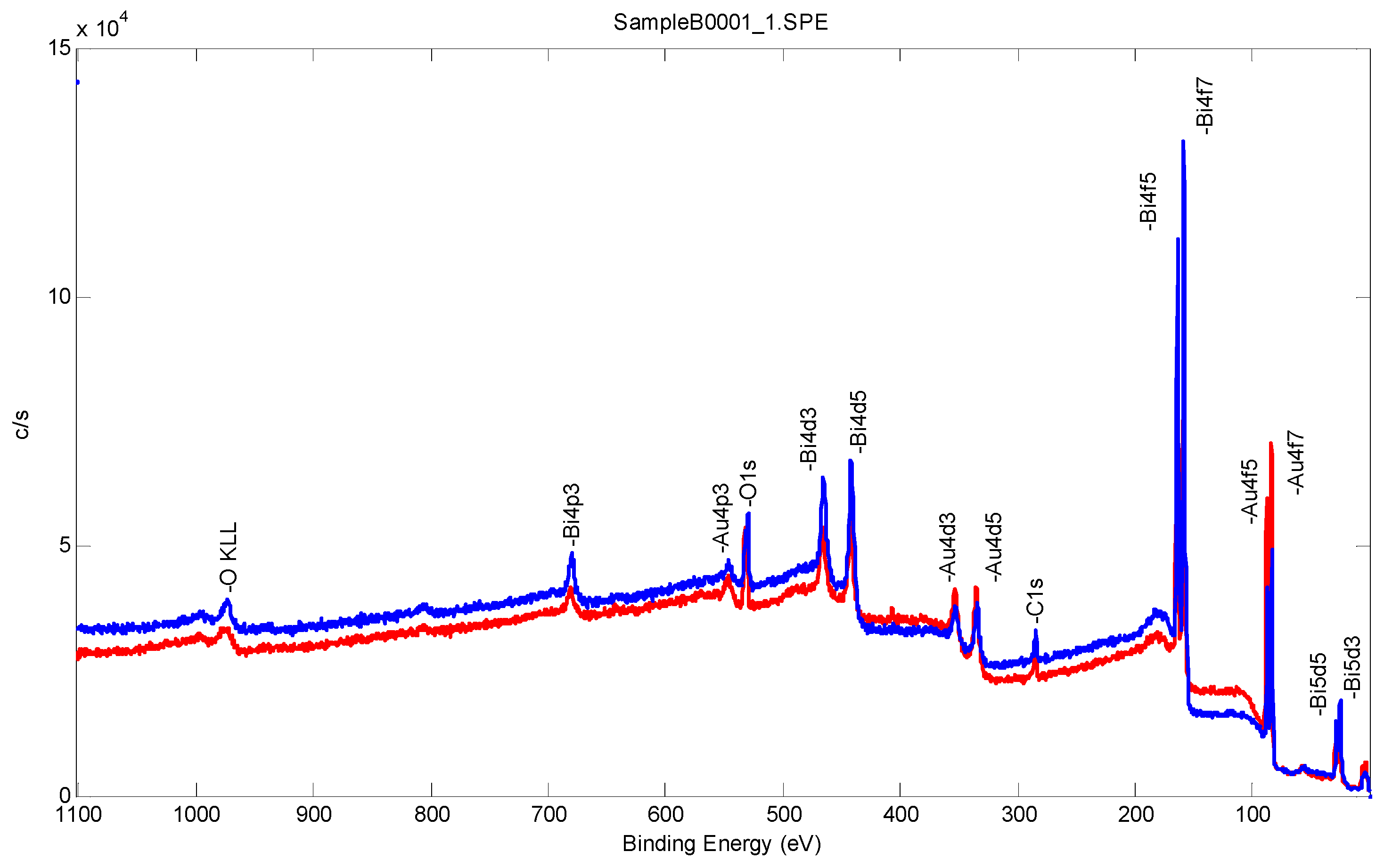
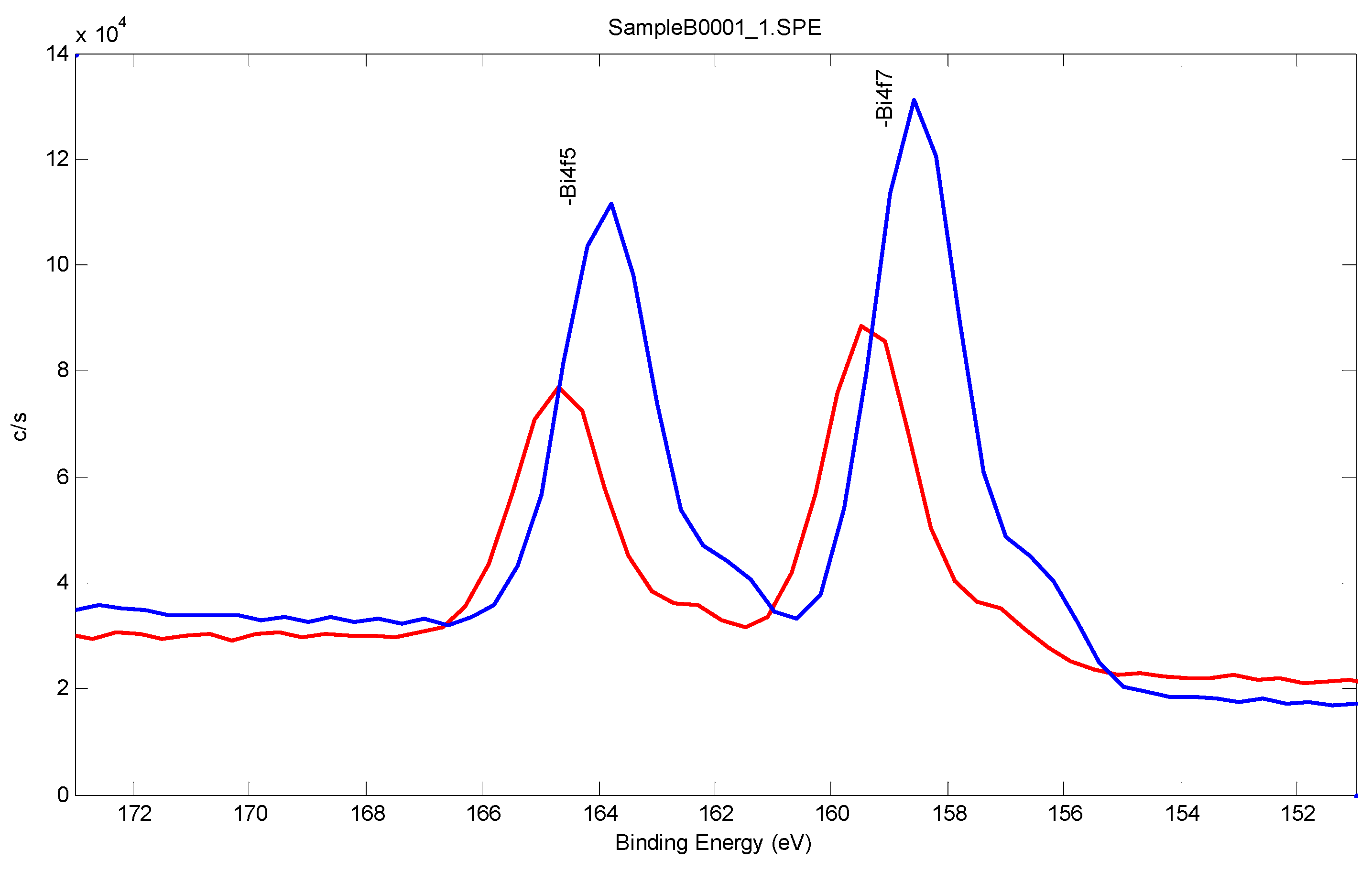
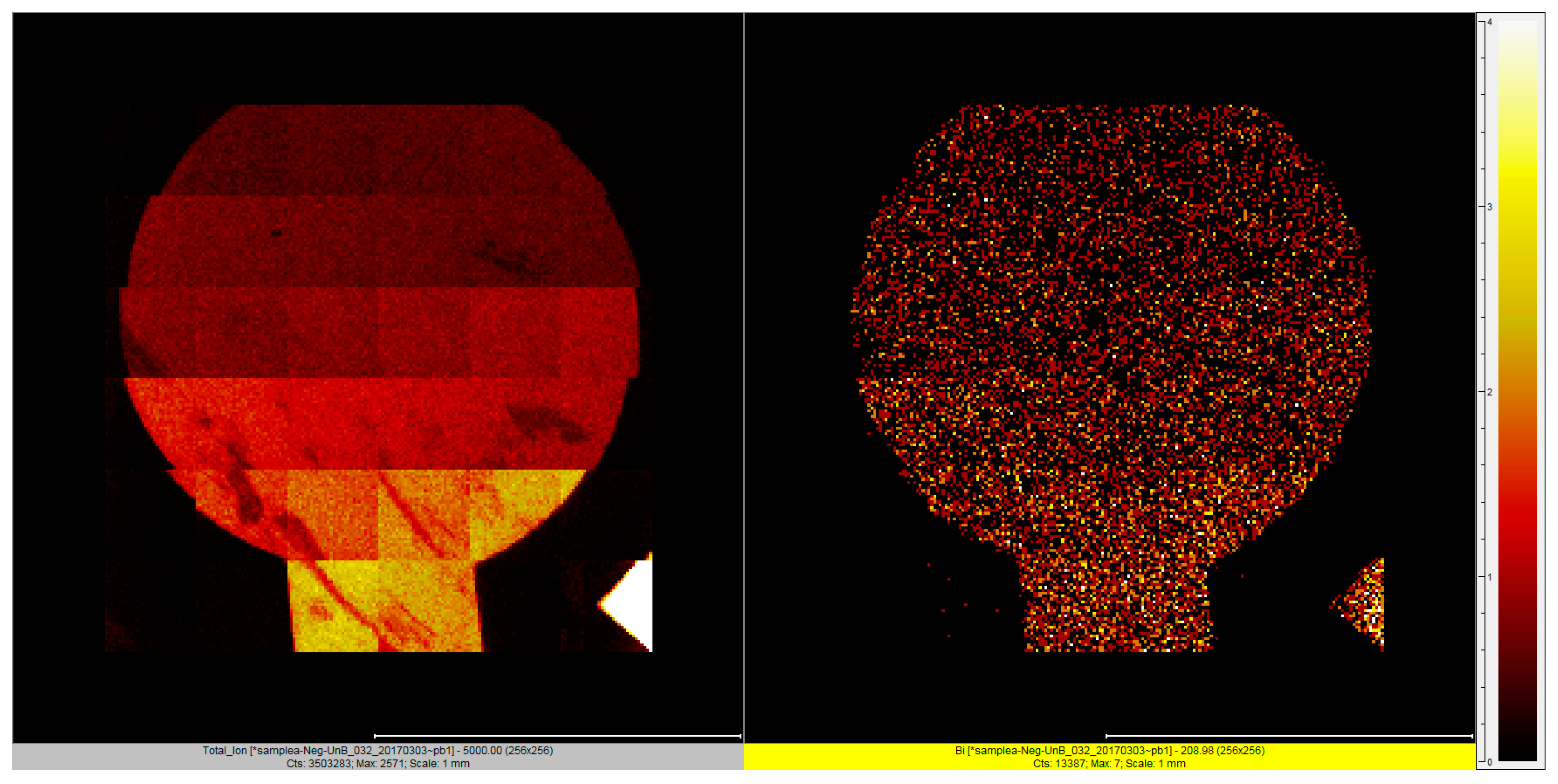
| Sample No. | C1s | O1s | Au4f | Bi4f |
|---|---|---|---|---|
| Sample A | 31.1 | 40.7 | 15.2 | 13.0 |
| Sample B | 27.5 | 40.5 | 10.3 | 21.8 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, Y.; Liu, C.C. A Simple, Cost-Effective Sensor for Detecting Lead Ions in Water Using Under-Potential Deposited Bismuth Sub-Layer with Differential Pulse Voltammetry (DPV). Sensors 2017, 17, 950. https://doi.org/10.3390/s17050950
Dai Y, Liu CC. A Simple, Cost-Effective Sensor for Detecting Lead Ions in Water Using Under-Potential Deposited Bismuth Sub-Layer with Differential Pulse Voltammetry (DPV). Sensors. 2017; 17(5):950. https://doi.org/10.3390/s17050950
Chicago/Turabian StyleDai, Yifan, and Chung Chiun Liu. 2017. "A Simple, Cost-Effective Sensor for Detecting Lead Ions in Water Using Under-Potential Deposited Bismuth Sub-Layer with Differential Pulse Voltammetry (DPV)" Sensors 17, no. 5: 950. https://doi.org/10.3390/s17050950






