Detection of Four Distinct Volatile Indicators of Colorectal Cancer using Functionalized Titania Nanotubular Arrays
Abstract
:1. Introduction
2. Background of Noninvasive Screening System for CRC
3. Materials and Methods
4. Results and Discussion
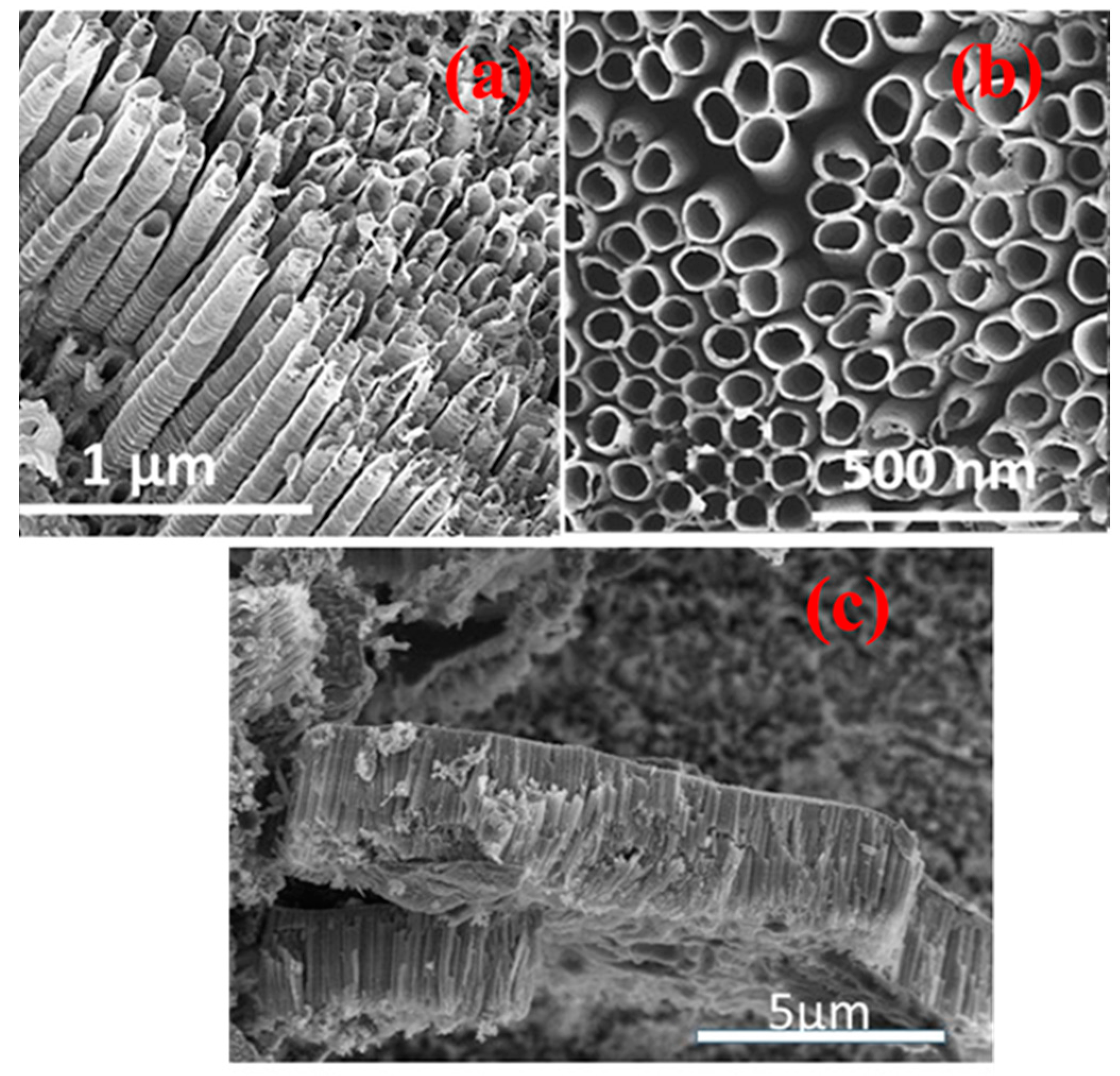
4.1. TNA Surface Characterization
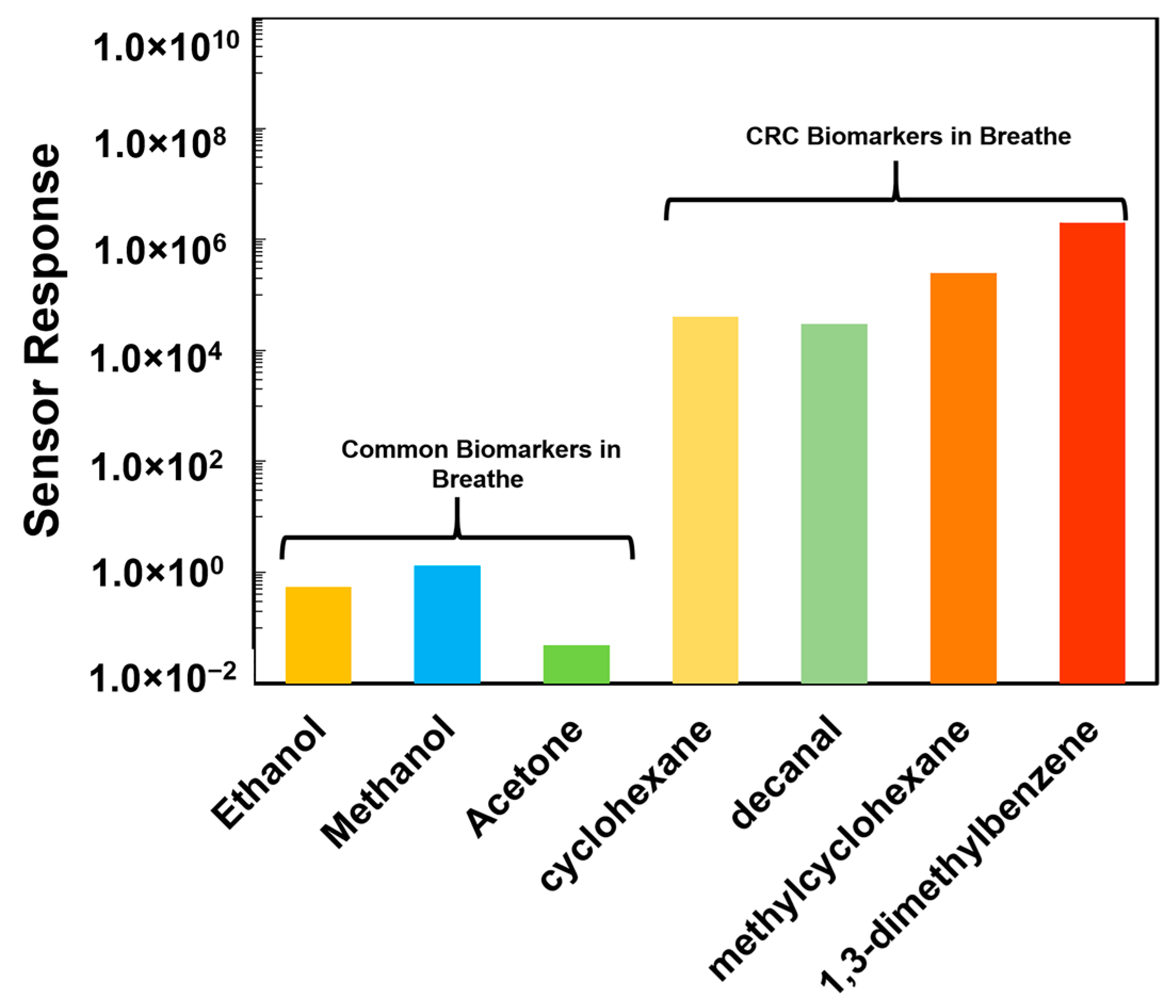
4.2. Detection of CRC Biomarkers with Ni-TNA Sensor
4.3. Sensing Mechanism




5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hirsch, F.R.; Franklin, W.A.; Gazdar, A.F.; Bunn, P.A. Early detection of lung cancer: Clinical perspectives of recent advances in biology and radiology. Clin. Cancer Res. 2001, 7, 5–22. [Google Scholar] [PubMed]
- Kyriakos Kalogerakos, N.B. Chrisostomos Sofoudis, Early breast cance: A review. Cancer Ther. 2008, 6, 463–476. [Google Scholar]
- Peng, G.; Hakim, M.; Broza, Y.Y.; Billan, S.; Abdah-Bortnyak, R.; Kuten, A.; Tisch, U.; Haick, H. Detection of lung, breast, colorectal, and prostate cancers from exhaled breath using a single array of nanosensors. Br. J. Cancer. 2010, 103, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Altomare, D.F.; Di Lena, M.; Porcelli, F.; Travaglio, E.; Longobardi, F.; Tutino, M.; Depalma, N.; Tedesco, G.; Sardaro, A.; Memeo, R.; et al. Effects of Curative Colorectal Cancer Surgery on Exhaled Volatile Organic Compounds and Potential Implications in Clinical Follow-up. Ann. Surg. 2015, 262, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Di Lena, M.; Porcelli, F.; Altomare, D.F. Volatile organic compounds as new biomarkers for colorectal cancer: A review. Colorectal Dis. 2016, 18, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Ahlquist, D.A.; Skoletsky, J.E.; Boynton, K.A.; Harrington, J.J.; Mahoney, D.W.; Pierceall, W.E.; Thibodeau, S.N.; Shuber, A.P. Colorectal cancer screening by detection of altered human DNA in stool: Feasibility of a multitarget assay panel. Gastroenterology 2000, 119, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, H.; Kohnoe, S.; Yamazato, T.; Satoh, Y.; Morizono, G.; Shikata, K.; Morita, M.; Watanabe, A.; Morita, M.; Kakeji, Y.; et al. Colorectal cancer screening with odour material by canine scent detection. Gut 2011, 60, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Arasaradnam, R.P.; McFarlane, M.J.; Ryan-Fisher, C.; Westenbrink, E.; Hodges, P.; Thomas, M.G.; Chambers, S.; O’Connell, N.; Bailey, C.; Harmston, C.; et al. Detection of Colorectal Cancer (CRC) by Urinary Volatile Organic Compound Analysis. PLoS ONE 2014, 9, e108750. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.; Podmore, I. Current Challenges in Volatile Organic Compounds Analysis as Potential Biomarkers of Cancer. J. Biomark. 2015, 2015, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Gatenby, R.A.; Gillies, R.J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer 2004, 4, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Vousden, K.H.; Ryan, K.M. p53 and metabolism. Nat. Rev. Cancer 2009, 9, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Okunieff, P.; Fenton, B.; Chen, Y. Past, present, and future of oxygen in cancer research. Adv. Exp. Med. Biol. 2005, 566, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Aksenov, A.A.; Gojova, A.; Zhao, W.; Morgan, J.T.; Sankaran, S.; Sandrock, C.E.; Davis, C.E. Characterization of Volatile Organic Compounds in Human Leukocyte Antigen Heterologous Expression Systems: A Cell’s “Chemical Odor Fingerprint”. ChemBioChem 2012, 13, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Hakim, M.; Broza, Y.Y.; Barash, O.; Peled, N.; Phillips, M.; Amann, A.; Haick, H. Volatile Organic Compounds of Lung Cancer and Possible Biochemical Pathways. Chem. Rev. 2012, 112, 5949–5966. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, P.; Lian, A.; Sun, B.; Wang, X.; Guo, L.; Chi, C.; Liu, S.; Zhao, W.; Luo, S.; et al. Blood volatile compounds as biomarkers for colorectal cancer. Cancer Biol. Ther. 2014, 15, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Westenbrink, E.; Arasaradnam, R.P.; O’Connell, N.; Bailey, C.; Nwokolo, C.; Bardhan, K.D.; Covington, J.A. Development and application of a new electronic nose instrument for the detection of colorectal cancer. Biosens. Bioelectron. 2015, 67, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.L.; Passos, M.; Câmara, J.S. Investigation of urinary volatile organic metabolites as potential cancer biomarkers by solid-phase microextraction in combination with gas chromatography-mass spectrometry. Br. J. Cancer 2011, 105, 1894–1904. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ke, C.; Wang, X.; Chi, C.; Guo, L.; Luo, S.; Guo, Z.; Xu, G.; Zhang, F.; Li, E. Noninvasive detection of colorectal cancer by analysis of exhaled breath. Anal. Bioanal. Chem. 2014, 406, 4757–4763. [Google Scholar] [CrossRef] [PubMed]
- Amal, H.; Leja, M.; Funka, K.; Lasina, I.; Skapars, R.; Sivins, A.; Ancans, G.; Kikuste, I.; Vanags, A.; Tolmanis, I.; et al. Breath testing as potential colorectal cancer screening tool. Int. J. Cancer 2016, 138, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Kronborg, O.; Fenger, C.; Olsen, J.; Jørgensen, O.D.; Søndergaard, O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet 1996, 348, 1467–1471. [Google Scholar] [CrossRef]
- De Haan, M.C.; van Gelder, R.E.; Graser, A.; Bipat, S.; Stoker, J. Diagnostic value of CT-colonography as compared to colonoscopy in an asymptomatic screening population: A meta-analysis. Eur. Radiol. 2011, 21, 1747–1763. [Google Scholar] [CrossRef] [PubMed]
- Zonta, G.; Anania, G.; Fabbri, B.; Gaiardo, A.; Gherardi, S.; Giberti, A.; Guidi, V.; Landini, N.; Malagù, C. Detection of colorectal cancer biomarkers in the presence of interfering gases. Sens. Actuators B Chem. 2015, 218, 289–295. [Google Scholar] [CrossRef]
- Malagù, C.; Fabbri, B.; Gherardi, S.; Giberti, A.; Guidi, V.; Landini, N.; Zonta, G. Chemoresistive Gas Sensors for the Detection of Colorectal Cancer Biomarkers. Sensors 2014, 14, 18982–18992. [Google Scholar] [CrossRef] [PubMed]
- De Meij, T.G.; Ben Larbi, I.; van der Schee, M.P.; Lentferink, Y.E.; Paff, T.; Terhaar sive Droste, J.S.; Mulder, C.J.; van Bodegraven, A.A.; de Boer, N.K. Electronic nose can discriminate colorectal carcinoma and advanced adenomas by fecal volatile biomarker analysis: Proof of principle study. Int. J. Cancer 2014, 134, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Mohanty, S.K.; Guruswamy, G.; Smith, Y.R.; Misra, M. Detection of Food Decay Products using Functionalized One-Dimensional Titania Nanotubular Arrays. IEEE Sens. Lett. 2017. [Google Scholar] [CrossRef]
- Ray, R.S.; Sarma, B.; Mohanty, S.; Prisbrey, K.; Misra, M. Assessment of metals in detection of TB biomarkers: Novel computational approach. Mater. Chem. Phys. 2015, 161, 1–8. [Google Scholar] [CrossRef]
- Albiter, E.; Valenzuela, M.A.; Alfaro, S.; Valverde-Aguilar, G.; Martínez-Pallares, F.M. Photocatalytic deposition of Ag nanoparticles on TiO2: Metal precursor effect on the structural and photoactivity properties. J. Saudi Chem. Soc. 2015, 19, 563–573. [Google Scholar] [CrossRef]
- Zhang, J.; Li, L.; Yan, T.; Li, G. Selective Pt Deposition onto the Face (110) of TiO2 Assembled Microspheres That Substantially Enhances the Photocatalytic Properties. J. Phys. Chem. C 2011, 115, 13820–13828. [Google Scholar] [CrossRef]
- Li, J.; Xu, J.; Dai, W.-L.; Fan, K. Dependence of Ag Deposition Methods on the Photocatalytic Activity and Surface State of TiO2 with Twistlike Helix Structure. J. Phys. Chem. C 2009, 113, 8343–8349. [Google Scholar] [CrossRef]
- Bhattacharyya, D.; Smith, Y.R.; Misra, M.; Mohanty, S.K. Electrochemical detection of methyl nicotinate biomarker using functionalized anodized titania nanotube arrays. Mater. Res. Express 2015, 2, 25002. [Google Scholar] [CrossRef]
- Hazra, A.; Bhowmik, B.; Dutta, K.; Chattopadhyay, P.P.; Bhattacharyya, P. Stoichiometry, Length, and Wall Thickness Optimization of TiO2 Nanotube Array for Efficient Alcohol Sensing. ACS Appl. Mater. Interfaces 2015, 7, 9336–9348. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, D. Functionalized Titania Nanotubes Arrays Based Sensors for Electrochemical Dtection of Volatile Organic Biomarkers; Univeristy of Utah: Salt Lake City, UT, USA, 2016. [Google Scholar]
- Hosseini, M.G.; Momeni, M.M.; Faraji, M. Highly Active Nickel Nanoparticles Supported on TiO2 Nanotube Electrodes for Methanol Electrooxidation. Electroanalysis 2010, 22, 2620–2625. [Google Scholar] [CrossRef]
- Smith, Y.R.; Bhattacharyya, D.; Mohanty, S.K.; Misra, M. Anodic Functionalization of Titania Nanotube Arrays for the Electrochemical Detection of Tuberculosis Biomarker Vapors. J. Electrochem. Soc. 2016, 163, B83–B89. [Google Scholar] [CrossRef]
- Alshammari, A.; Köckritz, A.; Kalevaru, V.N.; Bagabas, A.; Martin, A. Direct oxidation of cyclohexane to adipic acid using nano-gold catalysts. Appl. Petrochem. Res. 2012, 2, 61–67. [Google Scholar] [CrossRef]
- Sarkar, B.; Suman, S.; Tiwari, R.; Singha, R.K.; Ghosh, S.; Shankha Acharyya, S.; Konathala, L.N.S.K.; Pendem, C.; Mantri, K.; Bal, R. Pt nanoparticles supported on mesoporous ZSM-5: A potential catalyst for reforming of methane with carbon dioxide. IJC-A 2012, 51A, 1348–1353. [Google Scholar]
- Makgwane, P.R.; Ray, S.S. Efficient room temperature oxidation of cyclohexane over highly active hetero-mixed WO3/V2O5 oxide catalyst. Catal. Commun. 2014, 54, 118–123. [Google Scholar] [CrossRef]
- Ide, Y.; Kawamoto, N.; Bando, Y.; Hattori, H.; Sadakane, M.; Sano, T. Ternary modified TiO2 as a simple and efficient photocatalyst for green organic synthesis. Chem. Commun. 2013, 49, 3652. [Google Scholar] [CrossRef] [PubMed]
- Gaur, V.; Sharma, A.; Verma, N. Catalytic oxidation of toluene and m-xylene by activated carbon fiber impregnated with transition metals. Carbon 2005, 43, 3041–3053. [Google Scholar] [CrossRef]
- Yolcular, S.; Olgun, Ö. Ni/Al2O3 catalysts and their activity in dehydrogenation of methylcyclohexane for hydrogen production. Catal. Today 2008, 138, 198–202. [Google Scholar] [CrossRef]
- Li, Z.; Ding, D.; Liu, Q.; Ning, C.; Wang, X. Ni-doped TiO2 nanotubes for wide-range hydrogen sensing. Nanoscale Res. Lett. 2014, 9, 118. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, D.; Smith, Y.R.; Mohanty, S.K.; Misra, M. Titania Nanotube Array Sensor for Electrochemical Detection of Four Predominate Tuberculosis Volatile Biomarkers. J. Electrochem. Soc. 2016, 163, B206–B214. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, X.-L.; Wang, Z.-L.; Xu, J.-J.; Wang, H.-G.; Zhang, X.-B. Electrostatic induced stretch growth of homogeneous β-Ni(OH)2 on graphene with enhanced high-rate cycling for supercapacitors. Sci. Rep. 2014, 3669. [Google Scholar] [CrossRef] [PubMed]
- Frederic Cosandey, G.S.; Singhal, A. Materials and Processing Issues in Nanostructured Semiconductor Gas Sensors. JOM-E 2000, 52, 1–6. [Google Scholar]
- Sturm, J.M.; Göbke, D.; Kuhlenbeck, H.; Döbler, J.; Reinhardt, U.; Ganduglia-Pirovano, M.V.; Sauer, J.; Freund, H.-J. Partial oxidation of methanol on well-ordered V2O5(001)/Au(111) thin films. Phys. Chem. Chem. Phys. 2009, 11, 3290. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.-W.; Park, K.-S.; Heo, J.-H.; Park, J.-G.; Kim, D.-W.; Choi, K.J.; Lee, J.-H.; Hong, S.-H. Gas sensing properties of defect-controlled ZnO-nanowire gas sensor. Appl. Phys. Lett. 2008, 93, 263103. [Google Scholar] [CrossRef]
- Orzol, M.; Martin, I.; Kocisek, J.; Dabkowska, I.; Langer, J.; Illenberger, E. Bond and site selectivity in dissociative electron attachment to gas phase and condensed phase ethanol and trifluoroethanol. Phys. Chem. Chem. Phys. 2007, 9, 3424. [Google Scholar] [CrossRef] [PubMed]








© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhattacharyya, D.; Kumar, P.; Mohanty, S.K.; Smith, Y.R.; Misra, M. Detection of Four Distinct Volatile Indicators of Colorectal Cancer using Functionalized Titania Nanotubular Arrays. Sensors 2017, 17, 1795. https://doi.org/10.3390/s17081795
Bhattacharyya D, Kumar P, Mohanty SK, Smith YR, Misra M. Detection of Four Distinct Volatile Indicators of Colorectal Cancer using Functionalized Titania Nanotubular Arrays. Sensors. 2017; 17(8):1795. https://doi.org/10.3390/s17081795
Chicago/Turabian StyleBhattacharyya, Dhiman, Pankaj Kumar, Swomitra K. Mohanty, York R. Smith, and Mano Misra. 2017. "Detection of Four Distinct Volatile Indicators of Colorectal Cancer using Functionalized Titania Nanotubular Arrays" Sensors 17, no. 8: 1795. https://doi.org/10.3390/s17081795




