A Microneedle Functionalized with Polyethyleneimine and Nanotubes for Highly Sensitive, Label-Free Quantification of DNA
Abstract
:1. Introduction
2. Materials and Methods
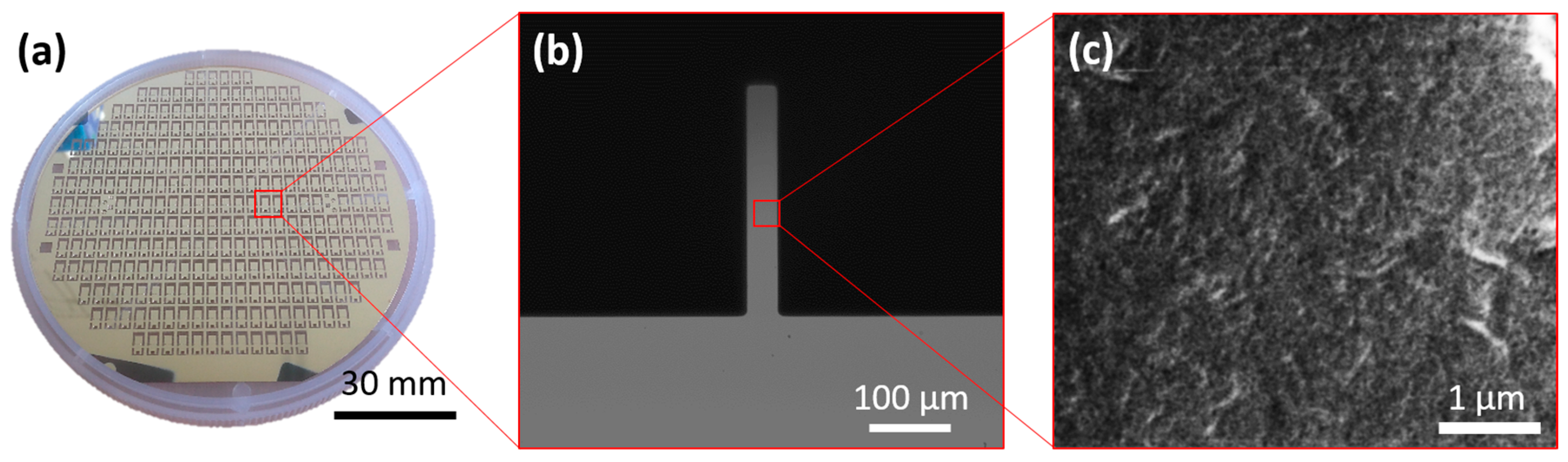
2.1. Microneedle Fabrication
2.2. Surface Functionalization and Characterization
2.3. Electrical Measurement
2.4. Sensitivity Test
3. Results and Discussions
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hyman, E.; Kauraniemi, P.; Hautaniemi, S.; Wolf, M.; Mousses, S.; Rozenblum, E.; Ringnér, M.; Sauter, G.; Monni, O.; Elkahloun, A.; et al. Impact of DNA amplification on gene expression patterns in breast cancer. Cancer Res. 2002, 62, 6240–6245. [Google Scholar] [PubMed]
- Brooker, R.J. Genetics: Analysis & Principles, 4th ed.; McGraw-Hill: New York, NY, USA, 2012. [Google Scholar]
- Miraglia, M.; Berdal, K.G.; Brera, C.; Corbisier, P.; Holst-Jensen, A.; Kok, E.J.; Marvin, H.J.P.; Schimmel, H.; Rentsch, J.; van Rie, J.P.P.F.; et al. Detection and traceability of genetically modified organisms in the food production chain. Food Chem. Toxicol. 2004, 42, 1157–1180. [Google Scholar] [CrossRef] [PubMed]
- Rouse, J.H.; Lillehei, P.T.; Sanderson, J.; Siochi, E.J. Polymer/single-walled carbon nanotube films assembled via donor–acceptor interactions and their use as scaffolds for silica deposition. Chem. Mater. 2004, 16, 3904–3910. [Google Scholar] [CrossRef]
- Ahn, S.J.; Costa, J.; Emanuel, J.R. Picogreen quantitation of DNA: Effective evaluation of samples pre- or post-PCR. Nucleic Acids Res. 1996, 24, 2623–2625. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, Y.; Zhang, L.; Cao, Y.; Li, Y.; Li, J.; Zhu, L.; Wu, G. Comparison of three common DNA concentration measurement methods. Anal. Biochem. 2014, 451, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Lieber, C.M. Nano-bioelectronics. Chem. Rev. 2016, 116, 215–257. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Sedeño, P.; Pingarrón, J.M.; Riu, J.; Rius, F.X. Electrochemical sensing based on carbon nanotubes. TrAC Trends Anal. Chem. 2010, 29, 939–953. [Google Scholar] [CrossRef]
- Tîlmaciu, C.-M.; Morris, M.C. Carbon nanotube biosensors. Front. Chem. 2015, 3, 59. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-X.; Choi, J.-W. Improved dispersion of carbon nanotubes in polymers at high concentrations. Nanomaterials 2012, 2, 329. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, Y.; Yamaguchi, H.; Einaga, N.; Esumi, M. Pitfalls of DNA quantification using DNA-binding fluorescent dyes and suggested solutions. PLoS ONE 2016, 11, e0150528. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.A. Label-Free Biosensors: Techniques and Applications; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Kong, J.; Franklin, N.R.; Zhou, C.; Chapline, M.G.; Peng, S.; Cho, K.; Dai, H. Nanotube molecular wires as chemical sensors. Science 2000, 287, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Heller, I.; Kong, J.; Heering, H.A.; Williams, K.A.; Lemay, S.G.; Dekker, C. Individual single-walled carbon nanotubes as nanoelectrodes for electrochemistry. Nano Lett. 2005, 5, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Ciszkowska, M.; Stojek, Z. Peer reviewed: Voltammetric and amperometric detection without added electrolyte. Anal. Chem. 2000, 72, 754A–760A. [Google Scholar] [CrossRef] [PubMed]
- Krapf, D.; Wu, M.-Y.; Smeets, R.M.M.; Zandbergen, H.W.; Dekker, C.; Lemay, S.G. Fabrication and characterization of nanopore-based electrodes with radii down to 2 nm. Nano Lett. 2006, 6, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Hiraiwa, M.; Lee, H.-B.; Lee, K.-H.; Cangelosi, G.A.; Chung, J.-H. Electrolyte-free amperometric immunosensor using a dendritic nanotip. RSC Adv. 2013, 3, 4281–4287. [Google Scholar] [CrossRef] [PubMed]
- Katz, E.; Willner, I. Probing biomolecular interactions at conductive and semiconductive surfaces by impedance spectroscopy: Routes to impedimetric immunosensors, DNA-sensors, and enzyme biosensors. Electroanalysis 2003, 15, 913–947. [Google Scholar] [CrossRef]
- Alvarez, N.; Ochmann, T.; Kienzle, N.; Ruff, B.; Haase, M.; Hopkins, T.; Pixley, S.; Mast, D.; Schulz, M.; Shanov, V. Polymer coating of carbon nanotube fibers for electric microcables. Nanomaterials 2014, 4, 879. [Google Scholar] [CrossRef] [PubMed]
- Shim, M.; Javey, A.; Shi Kam, N.W.; Dai, H. Polymer functionalization for air-stable n-type carbon nanotube field-effect transistors. J. Am. Chem. Soc. 2001, 123, 11512–11513. [Google Scholar] [CrossRef] [PubMed]
- Simbolo, M.; Gottardi, M.; Corbo, V.; Fassan, M.; Mafficini, A.; Malpeli, G.; Lawlor, R.T.; Scarpa, A. DNA qualification workflow for next generation sequencing of histopathological samples. PLoS ONE 2013, 8, e62692. [Google Scholar] [CrossRef] [PubMed]




| Method | Sensitivity | Label-Free | Processing Time | Sample Volume |
|---|---|---|---|---|
| Funtionalized microneedle | 3.2 fg/μL | Yes | 2 min | 5~10 μL |
| UV spectrophotometer | 2 ng/μL | Yes | 30 s | 0.5~2 μL |
| Fluorometer | 10 pg/μL | No | 5 min 20 s | 1~20 μL |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saadat-Moghaddam, D.; Kim, J.-H. A Microneedle Functionalized with Polyethyleneimine and Nanotubes for Highly Sensitive, Label-Free Quantification of DNA. Sensors 2017, 17, 1883. https://doi.org/10.3390/s17081883
Saadat-Moghaddam D, Kim J-H. A Microneedle Functionalized with Polyethyleneimine and Nanotubes for Highly Sensitive, Label-Free Quantification of DNA. Sensors. 2017; 17(8):1883. https://doi.org/10.3390/s17081883
Chicago/Turabian StyleSaadat-Moghaddam, Darius, and Jong-Hoon Kim. 2017. "A Microneedle Functionalized with Polyethyleneimine and Nanotubes for Highly Sensitive, Label-Free Quantification of DNA" Sensors 17, no. 8: 1883. https://doi.org/10.3390/s17081883





