A Fast Room Temperature NH3 Sensor Based on an Al/p-Si/Al Structure with Schottky Electrodes
Abstract
:1. Introduction
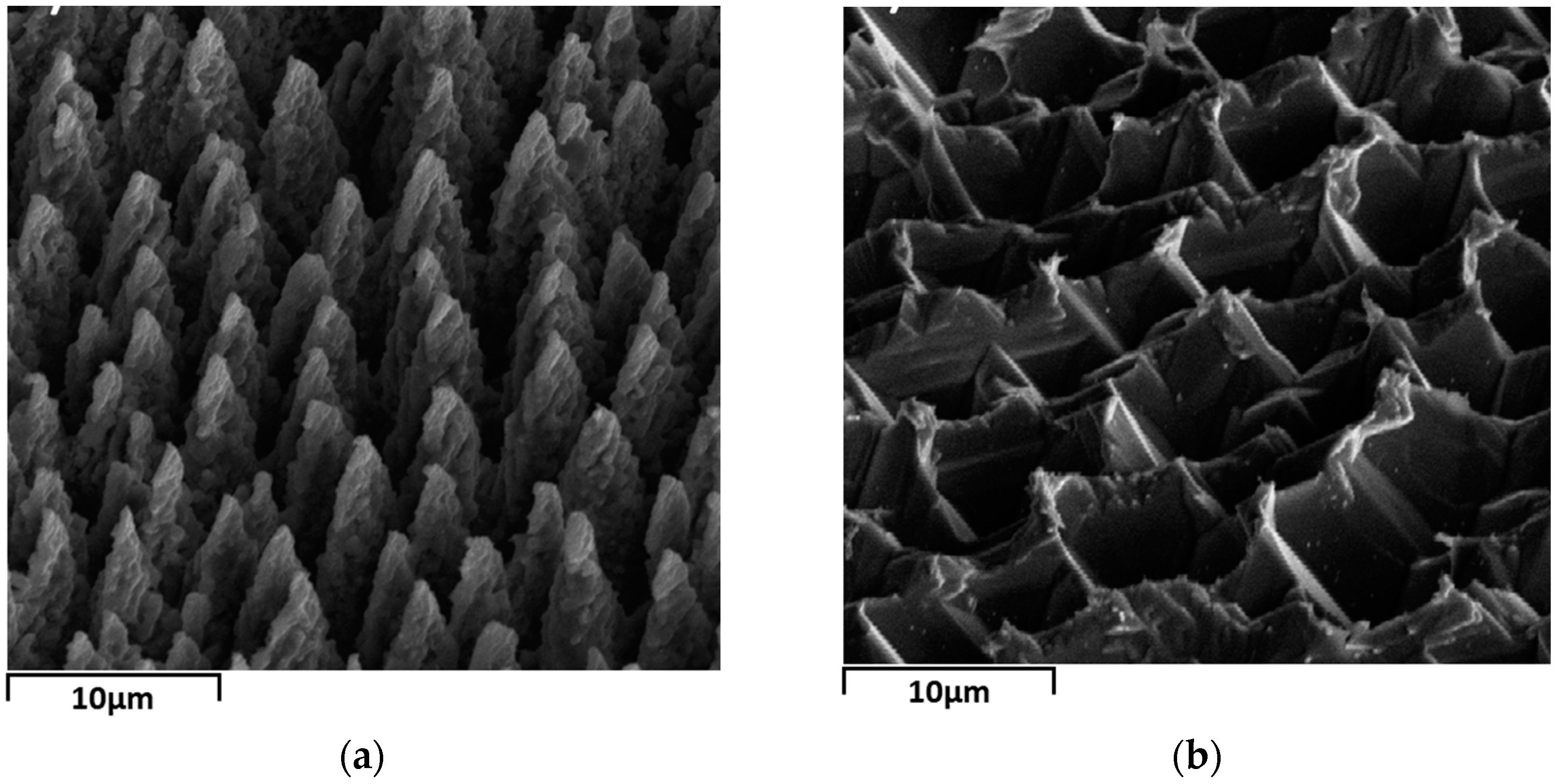
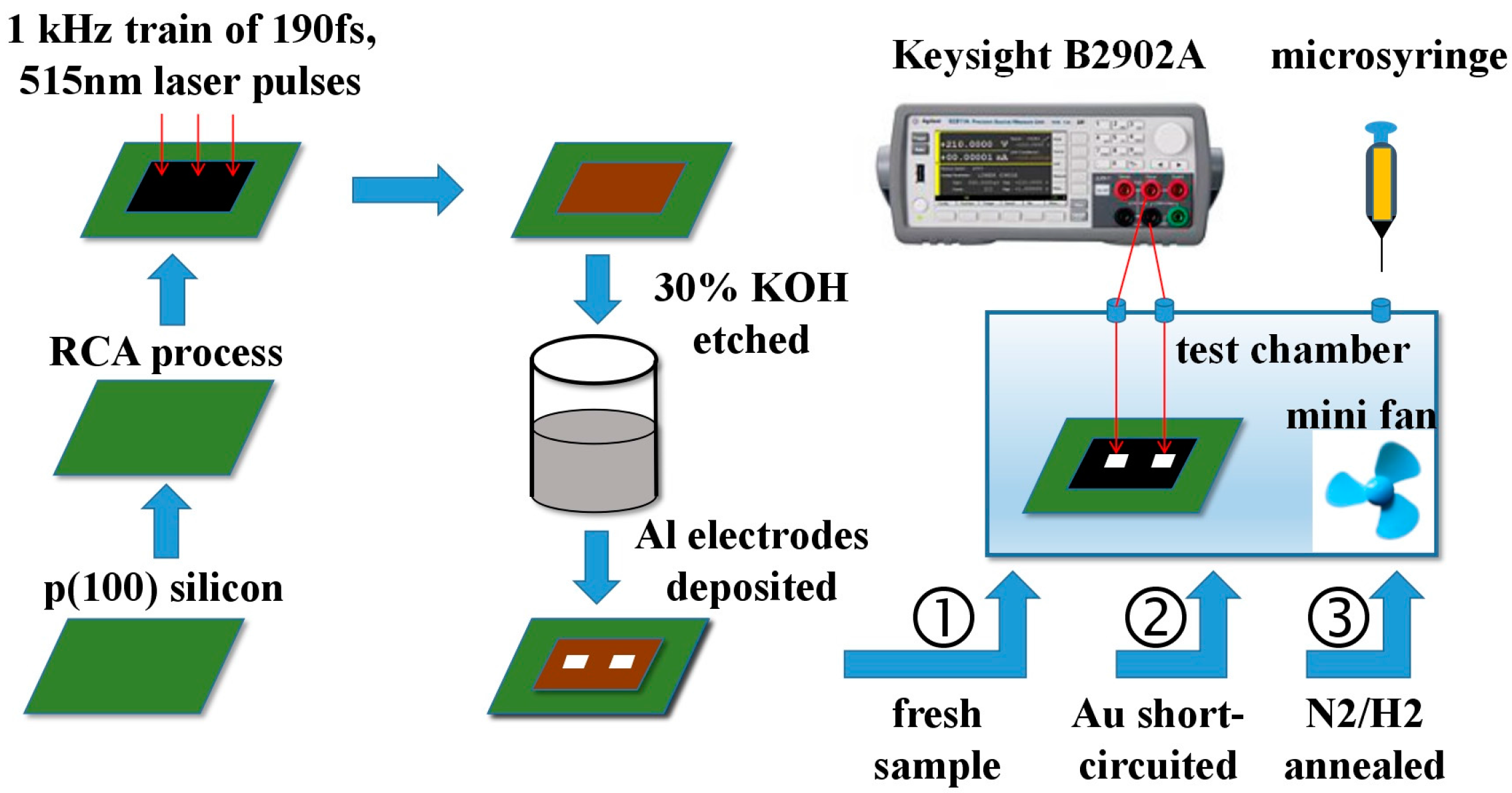
2. Materials and Methods
3. Results and Discussion
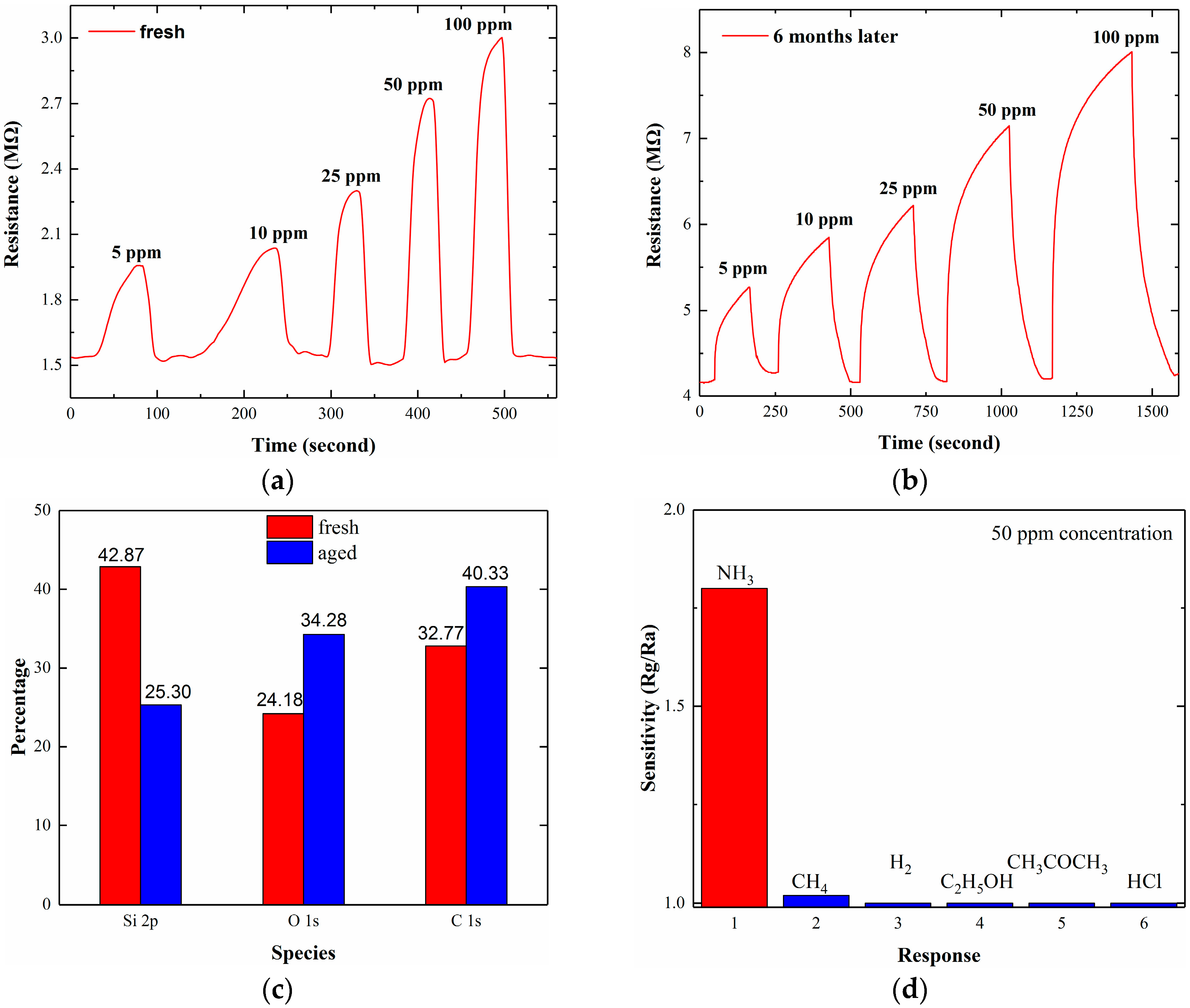
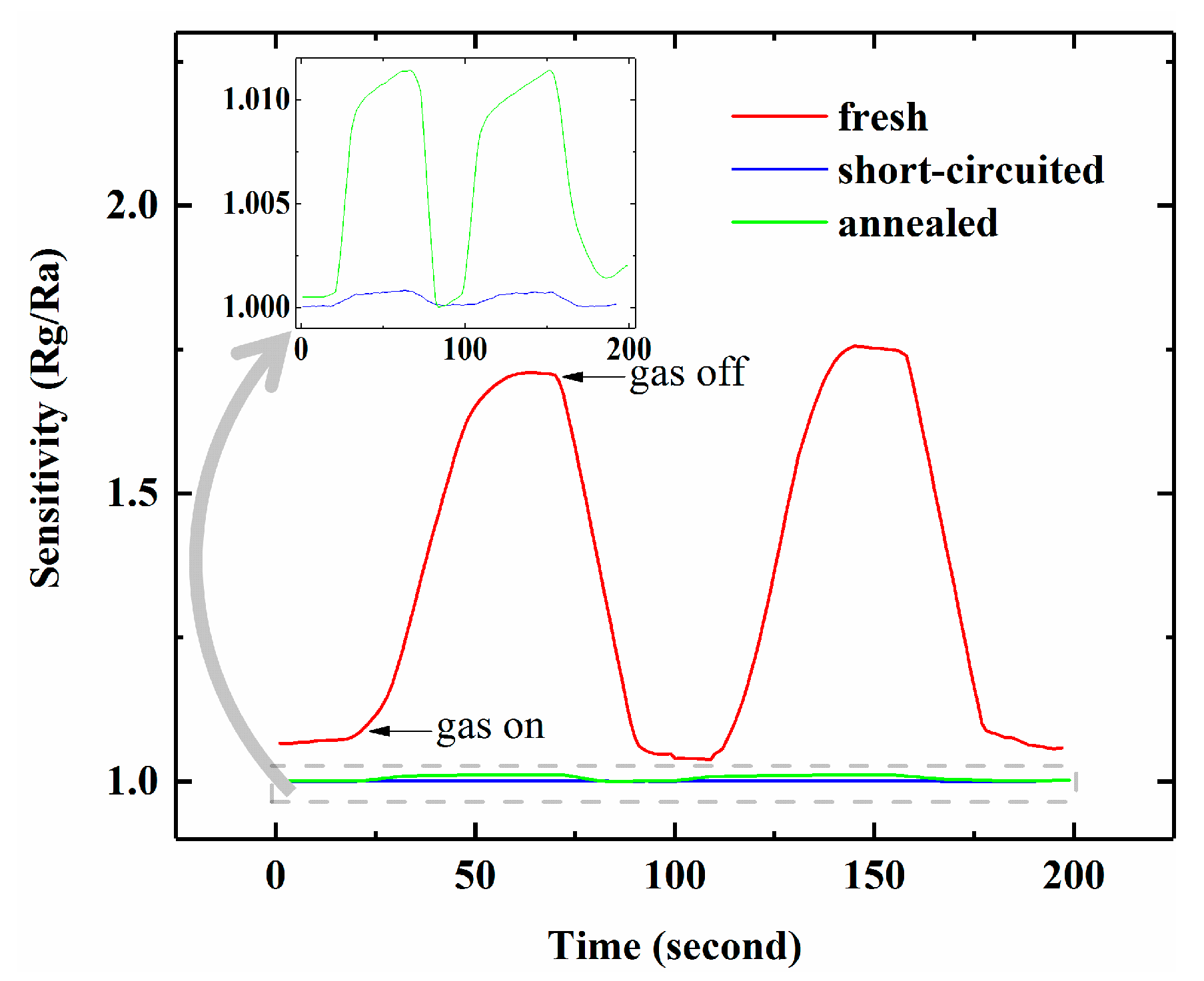
3.1. Dynamic NH3 Response
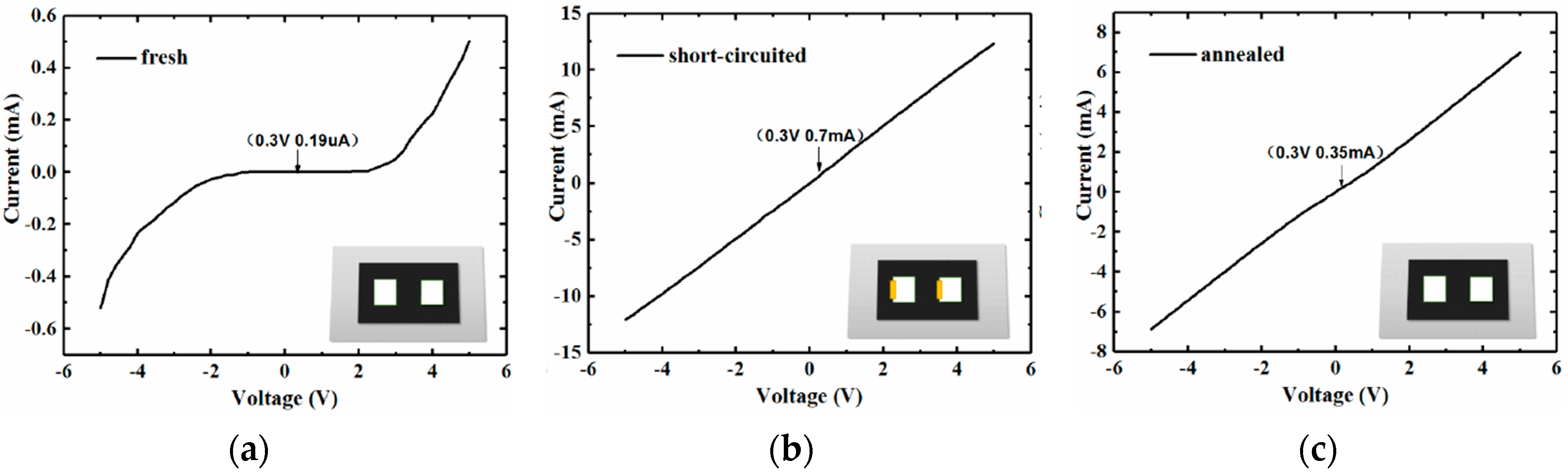
3.2. Improved Response with Schottky Electrodes
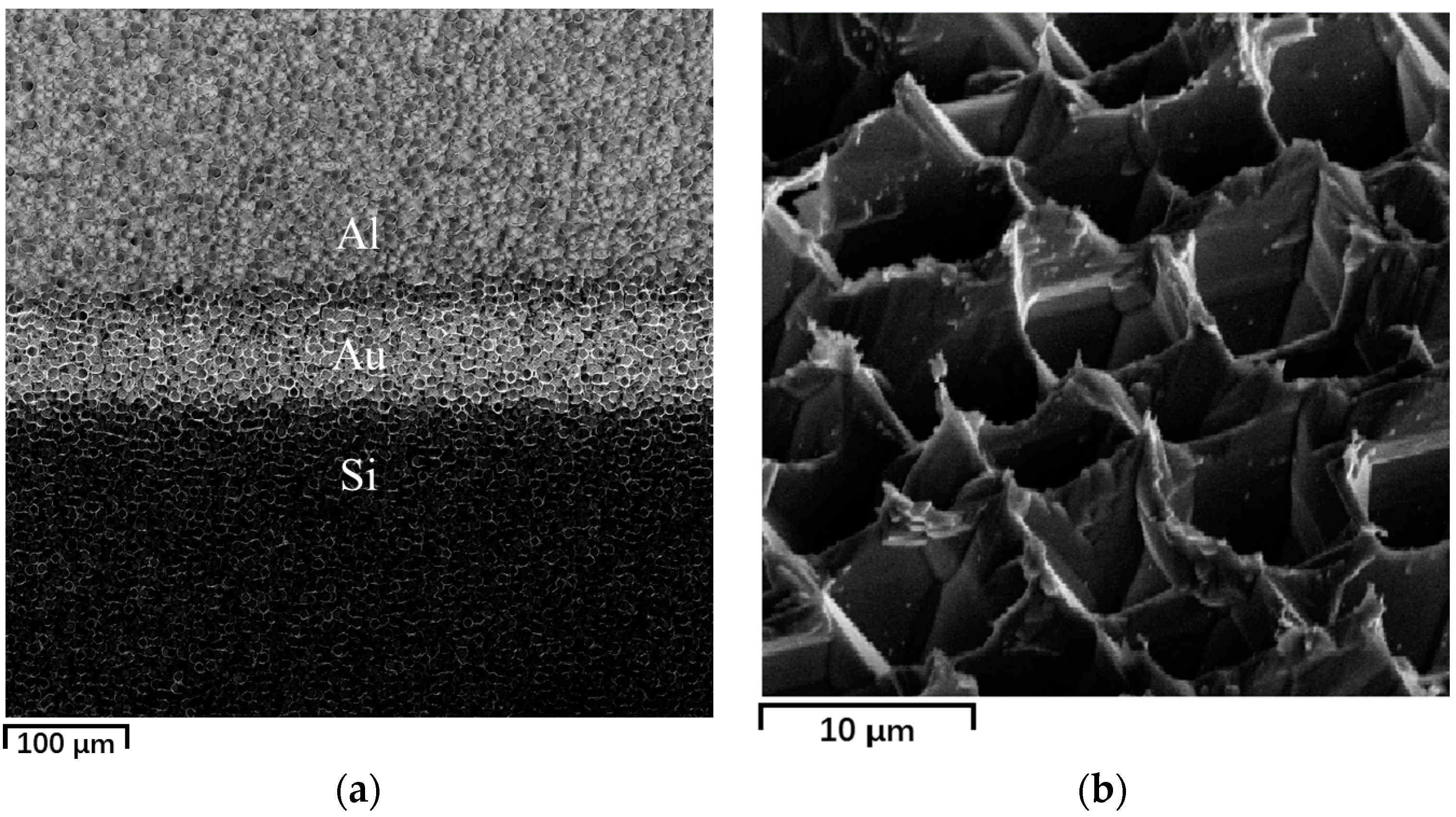
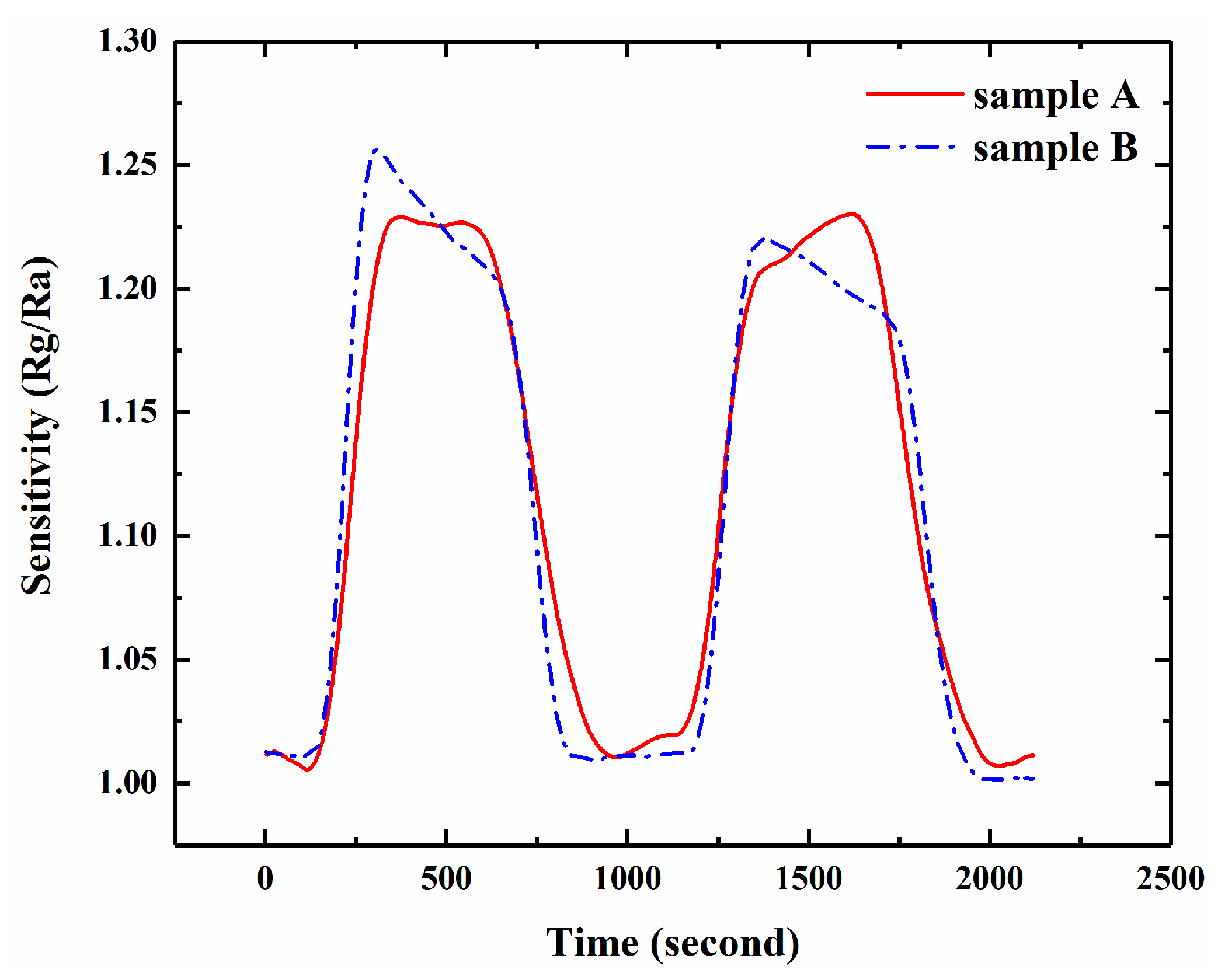
3.3. Effective Adsorption Sites
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Timmer, B.; Olthuis, W.; van den Berg, A. Ammonia sensors and their applications—A review. Sens. Actuators B Chem. 2005, 107, 666–677. [Google Scholar] [CrossRef]
- Eranna, G.; Joshi, B.C.; Runthala, D.P.; Gupta, R.P. Oxide materials for development of integrated gas sensors—A comprehensive review. Crit. Rev. Solid State 2004, 29, 111–188. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Brinzari, V.; Cho, B.K. Conductometric gas sensors based on metal oxides modified with gold nanoparticles: A review. Microchim. Acta 2016, 183, 1033–1054. [Google Scholar] [CrossRef]
- Mahendran, V.; Philip, J. An optical technique for fast and ultrasensitive detection of ammonia using magnetic nanofluids. Appl. Phys. Lett. 2013, 102, 063107. [Google Scholar] [CrossRef]
- Syrový, T.; Kuberský, P.; Sapurina, I.; Pretl, S.; Bober, P.; Syrová, L.; Hamácek, A.; Stejskal, J. Gravure-printed ammonia sensor based on organic polyaniline colloids. Sens. Actuators B Chem. 2016, 225, 510–516. [Google Scholar] [CrossRef]
- Gavgani, J.N.; Hasani, A.; Nouri, A.; Mahyari, M.; Salehi, A. Highly sensitive and flexible ammonia sensor based on S and N co-doped graphene quantum dots/polyaniline hybrid at room temperature. Sens. Actuators B Chem. 2016, 229, 239–248. [Google Scholar] [CrossRef]
- Kumar, L.; Rawal, I.; Kaur, A.; Annapoorni, S. Flexible room temperature ammonia sensor based on polyaniline. Sens. Actuators B Chem. 2017, 240, 408–416. [Google Scholar] [CrossRef]
- Bannov, A.G.; Jašek, O.; Manakhov, A.; Márik, M.; Nečas, D.; Zajíčková, L. High-performance ammonia gas sensors based on plasma treated carbon nanostructures. IEEE Sens. J. 2017, 7, 1964–1970. [Google Scholar] [CrossRef]
- Rana, M.M.; Ibrahim, D.S.; Asyraf, M.R.M.; Jarin, S.; Tomal, A. A review on recent advances of CNTs as gas sensors. In Sensor Review; Emerald Publishing Limited: Bingley, UK, 2017; Volume 19, pp. 127–136. [Google Scholar]
- Bannov, A.G.; Prášek, J.; Jašek, O.; Zajíčková, L. Investigation of pristine graphite oxide as room-temperature chemiresistive ammonia gas sensing material. Sensors 2017, 17, 320. [Google Scholar] [CrossRef] [PubMed]
- Karaduman, I.; Er, E.; Çelikkan, H.; Erk, N.; Acar, S. Room-temperature ammonia gas sensor based on reduced graphene oxide nanocomposites decorated by Ag, Au and Pt nanoparticles. J. Alloy. Compd. 2017, 722, 569–578. [Google Scholar] [CrossRef]
- Watanabe, K.; Okada, T.; Choe, I.; Sato, Y. Organic vapor sensitivity in a porous silicon device. Sens. Actuators B Chem. 1996, 33, 194–197. [Google Scholar] [CrossRef]
- Baratto, C.; Faglia, G.; Comini, E.; Sberveglieri, G.; Taroni, A.; La Ferrara, V.; Quercia, L.; Di Francia, G. A novel porous silicon sensor for detection of sub-ppm NO2 concentrations. Sens. Actuators B Chem. 2001, 77, 62–66. [Google Scholar] [CrossRef]
- Zad, A.I.; Rahimi, F.; Chavoshi, M.; Ahadian, M.M. Characterization of porous poly-silicon as a gas sensor. Sens. Actuators B Chem. 2004, 100, 341–346. [Google Scholar]
- Ozdemir, S.; Gole, J.L. The potential of porous silicon gas sensors. Curr. Opin. Solid State Mater. Sci. 2007, 11, 92–100. [Google Scholar] [CrossRef]
- Harraz, F.A. Porous silicon chemical sensors and biosensors: A review. Sens. Actuators B Chem. 2014, 202, 897–912. [Google Scholar] [CrossRef]
- Li, M.D.; Hu, M.; Zeng, P.; Ma, S.Y.; Yan, W.J.; Qin, Y.X. Effect of etching current density on microstructure and NH3-sensing properties of porous silicon with intermediate-sized pores. Electrochim. Acta 2013, 108, 167–174. [Google Scholar] [CrossRef]
- Lewis, S.E.; Deboer, J.R.; Gole, J.L.; Hesketh, P.J. Sensitive, selective, and analytical improvements to a porous silicon gas sensor. Sens. Actuators B Chem. 2005, 110, 54–65. [Google Scholar] [CrossRef]
- Yamamoto, N.; Tonomura, S.; Matsuoka, T.; Tsubomura, H. A study on a palladium-titanium oxide schottky diode as a detector for gaseous components. Surf. Sci. 1980, 92, 400–406. [Google Scholar] [CrossRef]
- Salehi, A.; Nikfarjam, A.; Kalantari, D.J. Highly sensitive humidity sensor using Pd/porous GaAs Schottky contact. IEEE Sens. J. 2006, 6, 1415–1421. [Google Scholar] [CrossRef]
- Luther, B.P.; Wolter, S.D.; Mohney, S.E. High temperature pt schottky diode gas sensors on n-type GaN. Sens. Actuators B Chem. 1999, 56, 164–168. [Google Scholar] [CrossRef]
- Stievenard, D.; Deresmes, D. Are electrical properties of an aluminum–porous silicon junction governed by dangling bonds? Appl. Phys. Lett. 1995, 67, 1570–1572. [Google Scholar] [CrossRef]
- Her, T.H.; Finlay, R.J.; Wu, C.; Deliwala, S.; Mazur, E. Microstructuring of silicon with femtosecond laser pulses. Appl. Phys. Lett. 1998, 73, 1673–1675. [Google Scholar] [CrossRef]
- Her, T.H.; Finlay, R.J.; Wu, C.; Mazur, E. Femtosecond laser-induced formation of spikes on silicon. Appl. Phys. Lett. 2000, 70, 383–385. [Google Scholar] [CrossRef]
- Kern, W. Handbook of Semiconductor Wafer Cleaning Technology: Science, Technology, and Applications; Noyes publications: Bergen, NY, USA, 1993; pp. 516–518. [Google Scholar]
- Bulgakov, A.V.; Ozerov, I.; Marine, W. Silicon clusters produced by femtosecond laser ablation: Non-thermal emission and gas-phase condensation. Appl. Phys. A 2004, 79, 1591–1594. [Google Scholar] [CrossRef] [Green Version]
- Crouch, C.H.; Carey, J.E.; Shen, M.; Mazur, E.; Genin, F.Y. Infrared absorption by sulfur-doped silicon formed by femtosecond laser irradiation. Appl. Phys. A 2004, 79, 1635–1641. [Google Scholar] [CrossRef]
- Crouch, C.H.; Carey, J.E.; Warrender, J.M.; Aziz, M.J.; Mazur, E.; Genin, F.Y. Comparison of structure and properties of femtosecond and nanosecond laser-structured silicon. Appl. Phys. Lett. 2004, 84, 1850–1852. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.; Li, N.; Zhu, Z.; Shao, H.Z.; Rong, X.M.; Liang, C.; Sun, H.B.; Feng, G.J.; Zhao, L.; Zhuang, J. A nitrogen-hyperdoped silicon material formed by femtosecond laser irradiation. Appl. Phys. Lett. 2014, 104, 091907. [Google Scholar] [CrossRef]
- Qin, Y.X.; Shen, W.J.; Li, X.; Hu, M. Effect of annealing on microstructure and NO2-sensing properties of tungsten oxide nanowires synthesized by solvothermal method. Sens. Actuators B Chem. 2011, 155, 646–652. [Google Scholar] [CrossRef]
- Pavlikov, A.V.; Osminkina, L.A.; Vorontsov, A.S.; Timoshenko, V.Y.; Kashkarov, P.K. Effect of ammonia adsorption on charge carriers in mesoporous silicon of n- and p-type conductivity. Phys. Status Solidi C 2007, 4, 2126–2130. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, J.H. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sens. Actuators B Chem. 2014, 192, 607–627. [Google Scholar] [CrossRef]
- Mawhinney, D.B.; Glass, J.A.; Yates, J.T. FTIR study of the oxidation of porous silicon. J. Phys. Chem. B 1997, 101, 1202–1206. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, S.; Liu, X.; Zhuang, J.; Zhao, L. A Fast Room Temperature NH3 Sensor Based on an Al/p-Si/Al Structure with Schottky Electrodes. Sensors 2017, 17, 1929. https://doi.org/10.3390/s17081929
Zhu S, Liu X, Zhuang J, Zhao L. A Fast Room Temperature NH3 Sensor Based on an Al/p-Si/Al Structure with Schottky Electrodes. Sensors. 2017; 17(8):1929. https://doi.org/10.3390/s17081929
Chicago/Turabian StyleZhu, Suwan, Xiaolong Liu, Jun Zhuang, and Li Zhao. 2017. "A Fast Room Temperature NH3 Sensor Based on an Al/p-Si/Al Structure with Schottky Electrodes" Sensors 17, no. 8: 1929. https://doi.org/10.3390/s17081929




