A New Hydrogen Sensor Based on SNS Fiber Interferometer with Pd/WO3 Coating
Abstract
:1. Introduction
2. Structure and Principle of Sensor
3. Simulation Analysis
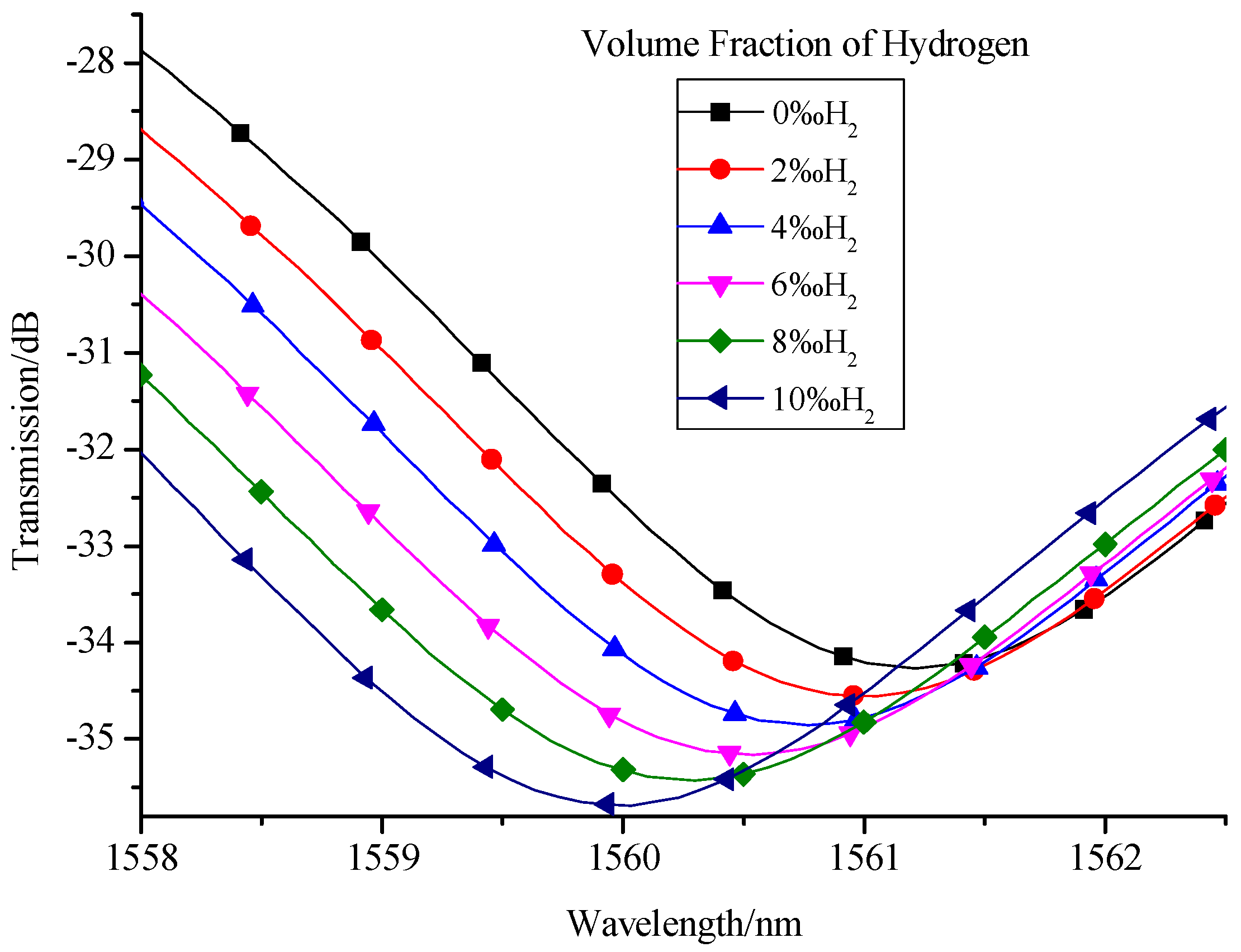
4. Experimental Test
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jacobson, M.Z.; Colella, W.G.; Golden, D.M. Clearing the air and improving health with hydrogen fuel-cell vehicles. Science 2005, 308, 1901–1905. [Google Scholar] [CrossRef] [PubMed]
- Dedes, E.K.; Hudson, D.A.; Turnock, S.R. Assessing the potential of hybrid energy technology to reduce exhaust emissions from global shipping. Energy Policy 2012, 40, 204–218. [Google Scholar] [CrossRef]
- Dincer, I. Renewable energy and sustainable development: A crucial review. Renew. Sustain. Energy Rev. 2000, 4, 157–175. [Google Scholar] [CrossRef]
- Silva, S.F.; Coelho, L.; Frazão, O. A review of palladium-based fiber-optic sensors for molecular hydrogen detection. IEEE Sens. J. 2012, 12, 93–102. [Google Scholar] [CrossRef]
- Hübert, T.; Boon-Brett, L.; Black, G.; Banach, U. Hydrogen sensors—A review. Sens. Actuators B Chem. 2011, 157, 329–352. [Google Scholar] [CrossRef]
- Firth, J.G.; Jones, A.; Jones, T.A. The principles of the detection of flammable atmospheres by catalytic devices. Combust. Flame 1973, 20, 303–311. [Google Scholar] [CrossRef]
- Pollak-Diener, G.; Obermeier, E. Heat-conduction microsensor based on silicon technology for the analysis of two-and three-component gas mixtures. Sens. Actuators B Chem. 1993, 13, 345–347. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Han, S.D.; Stetter, J.R. Review of electrochemical hydrogen sensors. Chem. Rev. 2009, 109, 1402–1433. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Zhu, L.; Wang, G. Optical fiber grating hydrogen sensors: A review. Sensors 2017, 17, 577. [Google Scholar] [CrossRef] [PubMed]
- Astbury, G.R.; Hawksworth, S.J. Spontaneous ignition of hydrogen leaks: A review of postulated mechanisms. Int. J. Hydrogen Energy 2007, 32, 2178–2185. [Google Scholar] [CrossRef]
- Yang, M.; Dai, J. Fiber optic hydrogen sensors: A review. Photonic Sens. 2014, 4, 300–324. [Google Scholar] [CrossRef]
- Butler, M.A. Micromirror optical-fiber hydrogen sensor. Sens. Actuators B Chem. 1994, 22, 155–163. [Google Scholar] [CrossRef]
- Tabib, A.M.; Sutapun, B.; Petrick, R. Highly sensitive hydrogen sensors using palladium coated fiber optics with exposed cores and evanescent field interactions. Sens. Actuators B Chem. 1999, 56, 158–163. [Google Scholar] [CrossRef]
- Bevenot, X.; Trouillet, A.; Veillas, C. Surface plasmon resonance hydrogen sensor using an optical fibre. Meas. Sci. Technol. 2001, 13, 118. [Google Scholar] [CrossRef]
- Butler, M.A.; Ginley, D.S. Hydrogen sensing with palladium-coated optical fibers. J. Appl. Phys. 1988, 64, 3706–3712. [Google Scholar] [CrossRef]
- Yang, M.; Sun, Y.; Zhang, D. Using Pd/WO3 composite thin films as sensing materials for optical fiber hydrogen sensors. Sens. Actuators B Chem. 2010, 143, 750–753. [Google Scholar] [CrossRef]
- Dai, J.; Yang, M.; Yu, X. Greatly etched fiber Bragg grating hydrogen sensor with Pd/Ni composite film as sensing material. Sens. Actuators B Chem. 2012, 174, 253–257. [Google Scholar] [CrossRef]
- Cai, L.; Zhao, Y.; Li, X.G. Applications of modal interferences in optical fiber sensors based on mismatch methods. Instrum. Sci. Technol. 2015, 43, 1–20. [Google Scholar] [CrossRef]
- Raghunandhan, R.; Chen, L.H.; Long, H.Y. Chitosan/PAA based fiber-optic interferometric sensor for heavy metal ions detection. Sens. Actuators B Chem. 2016, 233, 31–38. [Google Scholar] [CrossRef]
- Mohammed, W.S.; Mehta, A.; Johnson, E.G. Wavelength tunable fiber lens based on multimode interference. J. Lightwave Technol. 2004, 22, 469. [Google Scholar] [CrossRef]
- Zhao, Y.; Cai, L.; Li, X.G.; Meng, F.C.; Zhao, Z. Investigation of the high sensitivity RI sensor based on SMS fiber structure. Sens. Actuators A Phys. 2014, 205, 186–190. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.G.; Cai, L. A reflective intensity modulated fiber tilt angle sensor based on an all-photonic crystal fiber interferometer. Sens. Actuators A Phys. 2016, 244, 106–111. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Peng, H.; Zhou, T.; Zhang, L.; Zhang, Y.; Zhao, Y. Hydrogen sensor based on high-birefringence fiber loop mirror with sol-gel Pd/WO3 coating. Sens. Actuators B Chem. 2017, 248, 71–76. [Google Scholar] [CrossRef]
- Fardindoost, S.; Rahimi, F.; Ghasempour, R. Pd doped WO3 films prepared by sol-gel process for hydrogen sensing. Int. J. Hydrogen Energy 2010, 35, 854–860. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, X.; Liu, Y. Multi-point fiber-optic refractive index sensor by using coreless fibers. Opt. Commun. 2016, 365, 168–172. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Q.; Peng, H. Photonic crystal fiber modal interferometer with Pd/WO3 coating for real-time monitoring of dissolved hydrogen concentration in transformer oil. Rev. Sci. Instrum. 2016, 87, 125002. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.X.; Yang, M.H.; Huang, C.J.; Wang, G.P.; Dai, J.X.; Bai, W. Water photolysis effect on the long-term stability of fiber optic hydrogen sensor with Pt/WO3. Sci. Rep. 2016, 6, 39160. [Google Scholar] [CrossRef] [PubMed]
- Fardindoost, S.; Zad, A.I.; Hosseini, Z.S.; Hatamie, S. Detecting hydrogen using graphene quantum dots/WO3 thin films. Mater. Res. Express 2016, 3, 116407. [Google Scholar] [CrossRef]
- Dai, J.; Yang, M.; Yang, Z.; Li, Z.; Wang, Y.; Wang, G.; Zhang, Y.; Zhuang, Z. Performance of fiber Bragg grating hydrogen sensor coated with Pt-loaded WO3 coating. Sens. Actuators B Chem. 2014, 190, 657–663. [Google Scholar] [CrossRef]
- Coelho, L.; de Almeida, J.; Santos, J.L. Fiber optic hydrogen sensor based on an etched Bragg grating coated with palladium. Appl. Opt. 2015, 54, 10342–10348. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, D.N.; Yang, M.; Cheng, J.; Li, J. In-line Mach-Zehnder Interferometer and FBG with Pd film for simultaneous hydrogen and temperature detection. Sens. Actuators B Chem. 2014, 202, 893–896. [Google Scholar] [CrossRef]









© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, J.; Xie, W.; Song, X.; Zhang, Y. A New Hydrogen Sensor Based on SNS Fiber Interferometer with Pd/WO3 Coating. Sensors 2017, 17, 2144. https://doi.org/10.3390/s17092144
Shao J, Xie W, Song X, Zhang Y. A New Hydrogen Sensor Based on SNS Fiber Interferometer with Pd/WO3 Coating. Sensors. 2017; 17(9):2144. https://doi.org/10.3390/s17092144
Chicago/Turabian StyleShao, Jinxin, Wenge Xie, Xi Song, and Yanan Zhang. 2017. "A New Hydrogen Sensor Based on SNS Fiber Interferometer with Pd/WO3 Coating" Sensors 17, no. 9: 2144. https://doi.org/10.3390/s17092144




