A Gas Sensor Based on a Single SnO Micro-Disk
Abstract
:1. Introduction
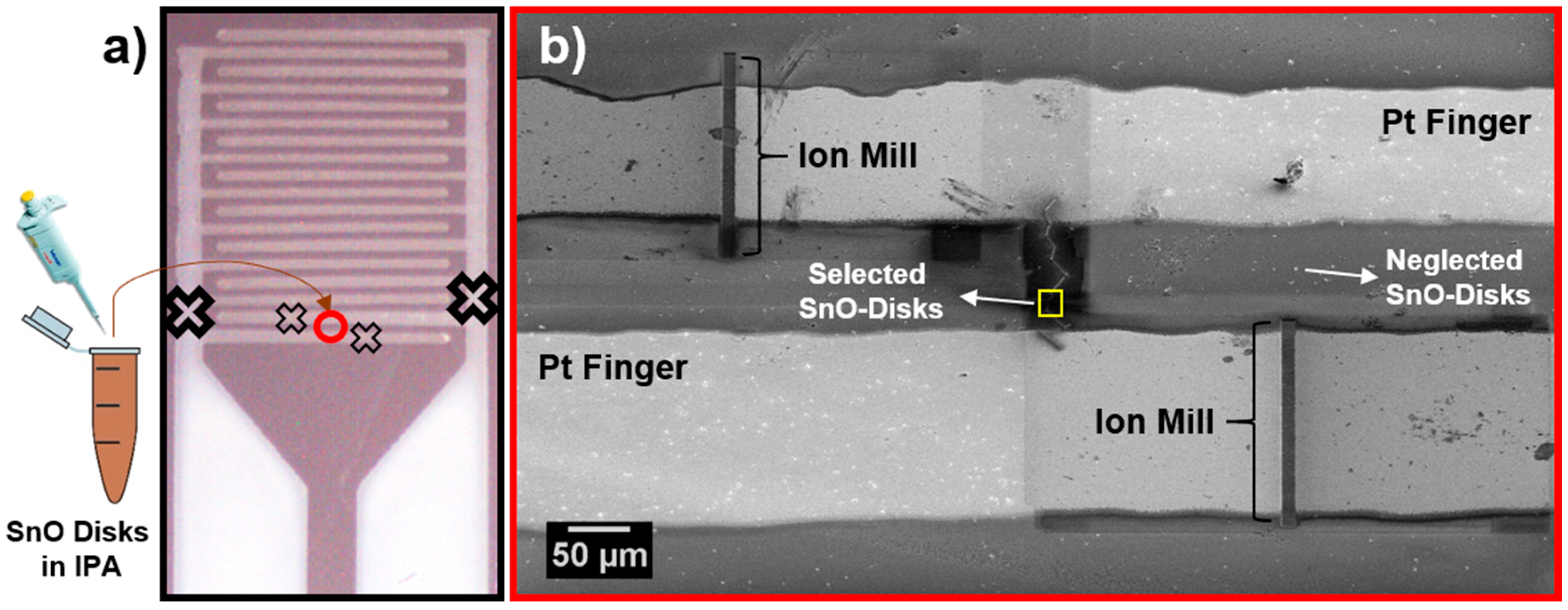
2. Materials and Methods
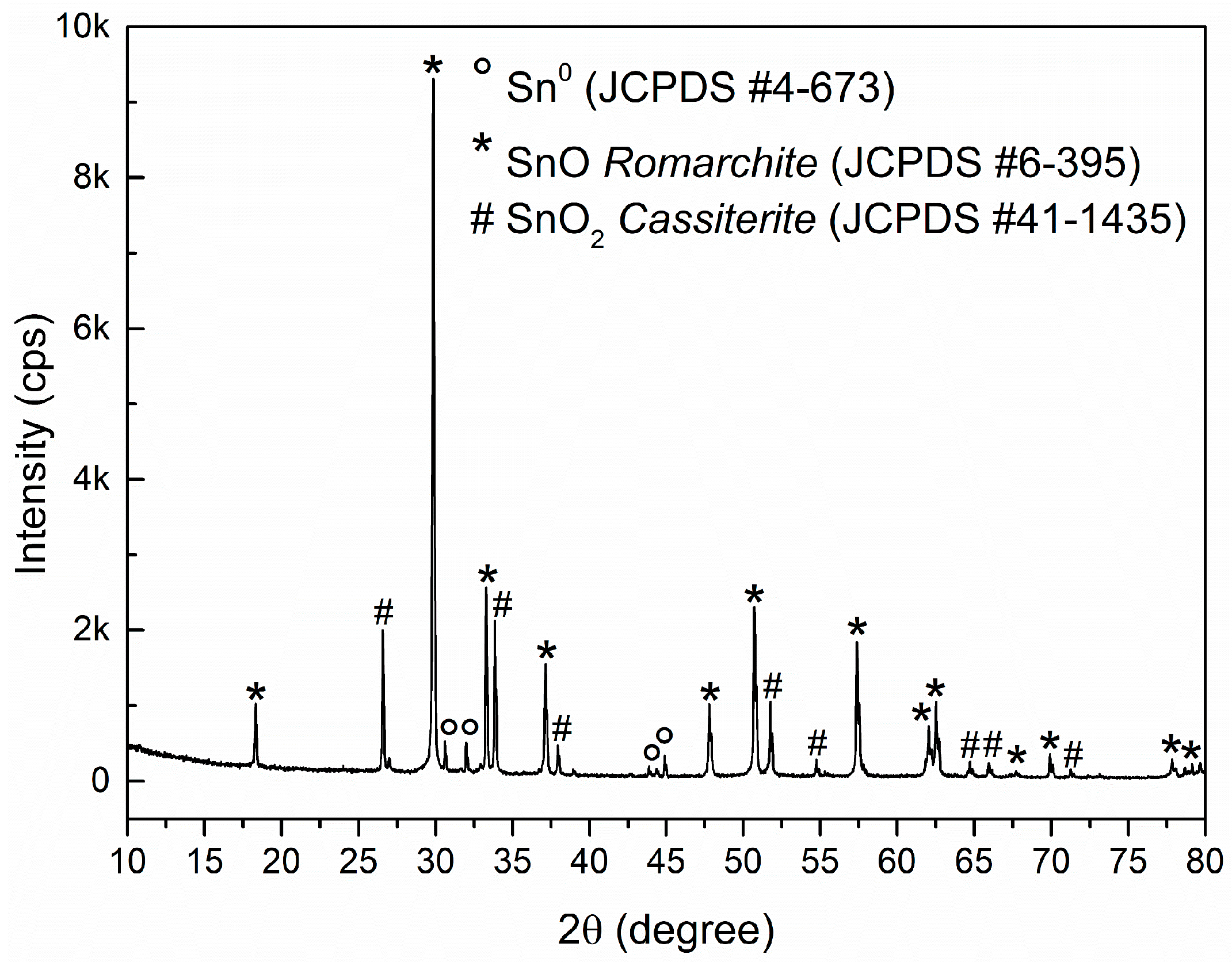
3. Results
4. Discussion
4.1. Temporal Differences in the Sensor Response Curves of Device 1
4.2. Comparison of the Sensor Response for Devices 1 and 2 When Exposed to NO2
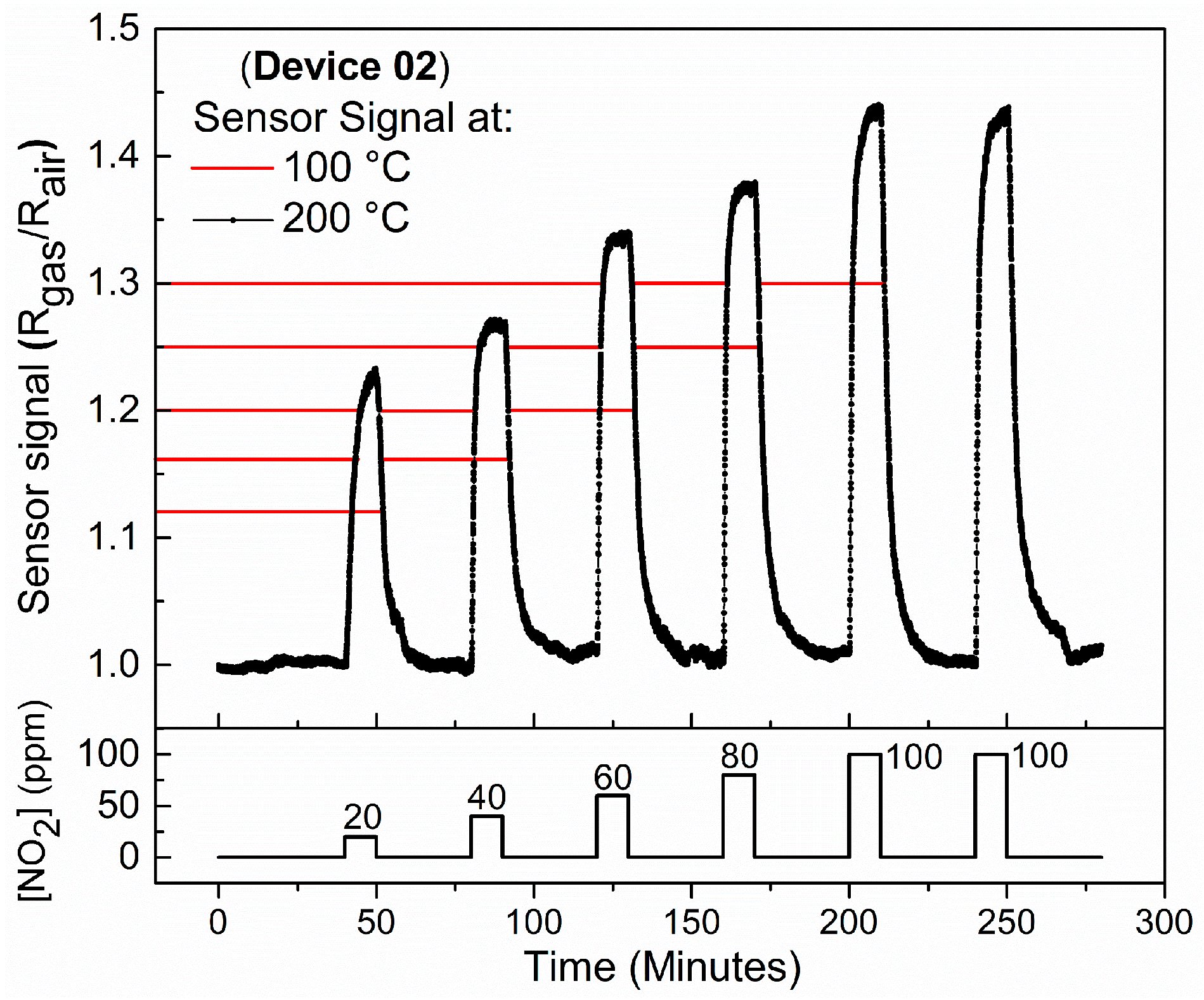
4.3. Temperature Influence on the Sensor Response of Device 2 When Exposed to NO2
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moss, R.; Sahner, K.; Fleischer, M.; Guth, U.; Barsan, N.; Weimar, U. Solid state gas sensor research in Germany–A status report. Sensors 2009, 9, 4323–4365. [Google Scholar] [CrossRef] [PubMed]
- Miura, N.; Kurosawa, H.; Hasei, M.; Lu, G.; Yamazoe, N. Stabilized zirconia-based sensor using oxide electrode for detection of NOx in high-temperature combustion-exhausts. Solid State Ion. 1996, 86–88, 1069–1073. [Google Scholar] [CrossRef]
- Zhuiykov, S.; Miura, N. Development of zirconia-based potentiometric NOx sensors for automotive and energy industries in the early 21st century: What are the prospects for sensors? Sens. Actuators B Chem. 2017, 121, 639–651. [Google Scholar] [CrossRef]
- Shelef, M.; Graham, G.W. Why rhodium in automotive three-way catalysts? Catal. Rev. 1994, 36, 433–457. [Google Scholar] [CrossRef]
- Ji, Y.; Toops, T.J.; Crocker, M. Effect of ceria on the storage and regeneration behavior of a model lean NOx trap catalyst. Catal. Lett. 2007, 119, 257–264. [Google Scholar] [CrossRef]
- Niyat, F.Y.; Sabzevar, I.; Abadi, M.H.S. The review of semiconductor gas sensor for NOx detecting. Turkish Online J. Des. Art Commun. 2016, 6, 898–937. [Google Scholar] [CrossRef]
- Batzill, M.; Diebold, U. The surface and materials science of tin oxide. Prog. Surf. Sci. 2005, 79, 47–154. [Google Scholar] [CrossRef]
- Chen, X.; Wong, C.K.Y.; Yuan, C.A.; Zhang, G. Nanowire-based gas sensors. Sens. Actuators B Chem. 2013, 177, 178–195. [Google Scholar] [CrossRef]
- Suman, P.H.; Felix, A.A.; Tuller, H.L.; Varela, J.A.; Orlandi, M.O. Comparative gas sensor response of SnO2, SnO and Sn3O4 nanobelts to NO2 and potential interferents. Sens. Actuators B Chem. 2015, 208, 122–127. [Google Scholar] [CrossRef]
- Liu, J.; Wang, C.; Yang, Q.; Gao, Y.; Zhou, X.; Liang, X.; Sun, P.; Lu, G. Hydrothermal synthesis and gas-sensing properties of flower-like Sn3O4. Sens. Actuators B Chem. 2016, 224, 128–133. [Google Scholar] [CrossRef]
- Li, X.; Wang, F.; Tu, J.; Shah, H.U.; Hu, J.; Li, Y.; Lu, Y.; Xu, M. Synthesis and ethanol sensing properties of novel hierarchical Sn3O4 nanoflowers. Nanomaterials 2015, 16. [Google Scholar] [CrossRef]
- Suman, P.H.; Felix, A.A.; Tuller, H.L.; Varela, J.A.; Orlandi, M.O. Giant chemo-resistance of SnO disk-like structures. Sens. Actuators B Chem. 2013, 186, 103–108. [Google Scholar] [CrossRef]
- Hernandez-Ramirez, F.; Prades, J.D.; Hackner, A.; Fischer, T.; Mueller, G.; Mathur, S.; Morante, J.R. Miniaturized ionization gas sensors from single metal oxide nanowires. Nanoscale 2011, 3, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Kolmakov, A.; Klenov, D.O.; Lilach, Y.; Stemmer, S.; Moskovits, M. Enhanced gas sensing by individual SnO2 nanowires and nanobelts functionalized with Pd catalyst particles. Nano Lett. 2005, 5, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Cardoza-Contretas, M.N.; Romo-Herrera, J.M.; Ríos, L.A.; García-Gutiérrez, R.; Zepada, T.A.; Contreras, O.E. Single ZnO nanowire-based gas sensors to detect low concentrations of hydrogen. Sensors 2015, 15, 30539–30544. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Ramirez, F.; Prades, J.D.; Jimenez-Diaz, R.; Fischer, T.; Romano-Rodriguez, A.; Mathur, S.; Morante, J.R. On the role of individual metal oxide nanowires in the scaling down of chemical sensors. Phys. Chem. Chem. Phys. 2009, 11, 7105–7110. [Google Scholar] [CrossRef] [PubMed]
- Ramgir, N.S.; Yang, Y.; Zacharias, M. Nanowire-Based Sensors. Small 2010, 6, 1705–1722. [Google Scholar] [CrossRef] [PubMed]
- Lupan, O.; Ursaki, V.V.; Chai, G.; Chow, L.; Emelchenki, G.A.; Tiginyanu, I.M.; Gruzintsev, A.N.; Redkin, A.N. Selective hydrogen gas nanosensor using individual ZnO nanowire with fast response at room temperature. Sens. Actuators B Chem. 2010, 144, 56–66. [Google Scholar] [CrossRef]
- Paulowicz, I.; Postica, V.; Lupan, O.; Wolff, N.; Shree, S.; Cojocaru, A.; Deng, M.; Mishra, Y.K.; Tiginyanu, I.; Kienle, L.; et al. Zinc oxide nanotetrapods with four different arm morphologies for versatile nanosensors. Sens. Actuators B Chem. 2018, 262, 425–435. [Google Scholar] [CrossRef]
- Kuang, Q.; Lao, C.; Wang, Z.L.; Xie, Z.; Zheng, L. High-sensitivity humidity sensor based on a single SnO2 nanowire. J. Am. Chem. Soc. 2007, 129, 6070–6071. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Ramirez, F.; Prades, J.D.; Morante, J.R. Metal oxide nanowire gas sensors. Sens. Mater. 2009, 21, 219–227. [Google Scholar]
- Tonezzer, M.; Hieu, N.V. Size-dependent response of single-nanowire gas sensors. Sens. Actuators B Chem. 2012, 163, 146–152. [Google Scholar] [CrossRef]
- Hernandez-Ramirez, F.; Prades, J.D.; Tarancon, A.; Barth, S.; Casals, O.; Jiménez-Diaz, R.; Pellicer, E.; Rodriguez, J.; Juli, M.A.; Romano-Rodriguez, A.; et al. Portable microsensor based on individual SnO2 nanowires. Nanotechnology 2007, 18, 495501. [Google Scholar] [CrossRef] [PubMed]
- Prades, J.D.; Jimenez-Diaz, R.; Hernandez-Ramirez, F.; Barth, S.; Cirera, A.; Romano-Rodriguez, A.; Mathur, S.; Morante, J.R. Ultralow power consumption gas sensors based on self-heated individual nanowires. Appl. Phys. Lett. 2008, 93, 123110. [Google Scholar] [CrossRef]
- Prades, J.D.; Jimenez-Diaz, R.; Hernandez-Ramirez, F.; Cirera, A.; Romano-Rodriguez, A.; Morante, J.R. Individual nanowire chemical sensor system self-powered with energy scavenging technologies. In Proceedings of the IEEE Solid-State Sensors, Actuators and Microsystems Conference, Denver, CO, USA, 21–25 June 2009; pp. 581–583. [Google Scholar]
- Gauzzi, F.; Verdini, B.; Maddalena, A.; Principi, G. X-ray diffraction and mössbauer analyses of SnO disproportionation products. Inorg. Chim. Acta 1985, 104, 1–7. [Google Scholar] [CrossRef]
- Gauzzi, F.; Verdini, B. Analysis of in-situ SnO disproportionation. J. Mater. Sci. Lett. 1985, 1492–1494. [Google Scholar] [CrossRef]
- Walsh, A.; Watson, G.W. Electronic structures of rocksalt, litharge, and herzenbergite SnO by density functional theory. Phys. Rev. B 2004, 70, 235114. [Google Scholar] [CrossRef]
- Walsh, A.; Payne, D.J.; Egdell, R.G.; Watson, G.W. Stereochemistry of post-transition metal oxides: Revision of the classical lone pair model. Chem. Soc. Rev. 2011, 40, 4455–4463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, J.; Kumar, R.; Bhatnagar, M.C. Effect of indium-doped SnO2 nanoparticles on NO2 gas sensing properties. Sens. Actuators B Chem. 2007, 126, 478–484. [Google Scholar] [CrossRef]
- Sharma, A.; Tomar, M.; Gupta, V. WO3 nanoclusters-SnO2 film gas sensor heterostructure with enhanced response for NO2. Sens. Actuators B Chem. 2007, 176, 675–684. [Google Scholar] [CrossRef]
- Vila, A.; Hernandez-Ramirez, F.; Rodriguez, J.; Casal, O.; Romano-Rodriguez, A.; Morante, J.R.; Abid, M. Fabrication of metallic contacts to nanometre-sized materials using a focused ion beam (FIB). Mater. Sci. Eng. 2006, 26, 1063–1066. [Google Scholar] [CrossRef]
- Hernandez-Ramirez, F.; Rodriguez, J.; Casals, O.; Russinyol, E.; Vila, A.; Romano-Rodriguez, A.; Morante, J.R.; Abid, M. Characterization of metal-oxide nanosensors fabricated with focused ion beam (FIB). Sens. Actuators B Chem. 2006, 118, 198–203. [Google Scholar] [CrossRef]
- Liewhiran, C.; Tamaekong, N.; Wisitsiraat, A.; Phanichphant, S. Highly selective environmental sensors based on flame-spray-made SnO2 nanoparticles. Sens. Actuators B Chem. 2012, 163, 51–60. [Google Scholar] [CrossRef]
- Solymosi, F.; Kiss, J. Adsorption and reduction of NO on tin (IV) oxide catalysts. J. Catal. 1976, 41, 202–211. [Google Scholar] [CrossRef]
- Maiti, A.; Rodriguez, J.A.; Law, M.; Kung, P.; McKiney, J.R.; Yang, P. SnO2 nanoribbons as NO2 sensors: Insights from first principles calculations. Nano Lett. 2003, 3, 1025–1028. [Google Scholar] [CrossRef]
- Lenaerts, S.; Roggen, J.; Maes, G. FT-IR characterization of tin dioxide gas sensor materials under working conditions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1995, 51, 883–894. [Google Scholar] [CrossRef]
- Gopel, W.; Schierbaum, K.D. SnO2 sensors: Current status and future prospects. Sens. Actuators B Chem. 1995, 26, 1–12. [Google Scholar] [CrossRef]
- Barsan, N.; Weimar, U. Conduction model of metal oxide gas sensors. J. Electroceramics. 2001, 7, 143–167. [Google Scholar] [CrossRef]
- Schipani, F.; Miller, D.R.; Ponce, M.A.; Aldao, C.M.; Akbar, S.A.; Morris, P.A.; Xu, J.C. Conduction mechanisms in SnO2 single-nanowire gas sensors: An impedance spectroscopy study. Sens. Actuators B Chem. 2017, 241, 99–108. [Google Scholar] [CrossRef]
- Weisz, P.B. Effects of electronic charge transfer between adsorbate and solid on chemisorption and catalysis. J. Chem. Phy. 1953, 21, 1531–1538. [Google Scholar] [CrossRef]
- Gurlo, A. Interplay between O2 and SnO2: Oxygen ionosorption and spectroscopic evidence for adsorbed oxygen. ChemPhysChem 2006, 7, 2041–2052. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhu, L.F.; Yang, Y.H.; Xu, N.S.; Yang, W. Fabrication of a SnO2 nanowire gas sensor and sensor performance for hydrogen. J. Phys. Chem. C 2008, 112, 6643–6647. [Google Scholar] [CrossRef]
- Starke, T.K.H.; Coles, G.S.V. Reduced response times using adsorption kinetics and pulsed-mode operation for the detection of oxides of nitrogen with nanocrystalline SnO2 sensors. IEEE Sens. J. 2003, 3, 447–453. [Google Scholar] [CrossRef]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masteghin, M.G.; Orlandi, M.O. A Gas Sensor Based on a Single SnO Micro-Disk. Sensors 2018, 18, 3229. https://doi.org/10.3390/s18103229
Masteghin MG, Orlandi MO. A Gas Sensor Based on a Single SnO Micro-Disk. Sensors. 2018; 18(10):3229. https://doi.org/10.3390/s18103229
Chicago/Turabian StyleMasteghin, Mateus G., and Marcelo O. Orlandi. 2018. "A Gas Sensor Based on a Single SnO Micro-Disk" Sensors 18, no. 10: 3229. https://doi.org/10.3390/s18103229





