3.1. Ink Composition Development
The most challenging step throughout the fabrication process of such a device as described in the introductory part is the formulation of the ink. It is necessary to consider that the parameters of the final printed layer have to meet all necessary chemical and physicochemical criteria to assure the compatibility of the ink with the substrate, the jetting performance and the storage stability of the suspension while preparing the ink composition. It is a multidimensional parameter space that must be researched and in which an optimum between all (and sometimes contradictory) requirements imposed on the material and its processing with the given equipment must be found. The crucial parameters enabling optimal printability are therefore ink density, viscosity, surface tension, the stability of the suspension and the size of the nanoparticles, which is partially related to the final resistance and the response of the sensing layer [
16,
25]. The key properties determining good processability by the used ink-jet printer Dimatix DMP-2800 Series are listed by the producer for disposable cartridges with printing heads as follows. The ink fluid viscosity shall be kept in a range of 10–12 mPa·s and its surface tension in a range of 28–42 mN·m
−1. Furthermore, it is suggested to use a drop velocity in a range of 7–9 m∙s
−1 as the first guess [
26], however, according to literature and experience in this study, a fluid ejection velocity of about 6 m∙s
−1 is commonly used. It is also recommended to filter all fluids to 0.2 μm, because particles bigger than 1/100 of the nozzle diameter may cause nozzle clogging. The first two parameters may be substantially changed by the ink composition and the size of the nanoparticles must be chosen to be smaller than the critical level. The processing parameters, namely the fluid ejection velocity and the droplet formation, are controlled by using waveform; however, they can be only slightly varied in comparison with the relatively free choice of the ink viscosity and the surface energy. According to the producer, the viscosity range may be extended from 1 mPa·s for water-like fluids up to 30 mPa·s which is declared as the highest viable for the printer. Similarly, a surface tension of about 70 mN·m
−1 represents the upper limit for printing with a given machine while the lower limit is 20 mN·m
−1. The original ink composition was experimentally developed within this space of parameters by the trial-error method of changing and alternating always only one variable until a satisfactory performance of the process was achieved. The properties of developed inks with variable nanoparticle loading are listed in
Table 1. The surface tension of the used ink compositions is compatible with the surface characteristics of the chosen substrate. The viscosity and the surface tension under given dynamic conditions did not result in the optimum formation of single droplets only, but the system worked in the regime of the one satellite droplet formation that merges with the main droplet during its flight before hitting the surface. Although this is a suboptimum process, it yielded good printing quality while other parameters were relatively easily kept at sufficient levels too.
The stability of the prepared nanodispersions represents another extremely important issue for their application as inks. Sufficient shelf life is a great advantage and at least easy redisperseability must be assured for any practical utilization of inks. Due to the above-mentioned requirements the optimal amount and ratio of the two additives was determined based on the critical micelle concentration in an aqueous medium. The surface tension was controlled by using a wetting agent which produces a significant decrease in the surface tension of the aqueous system and therefore particularly improves substrate wetting and levelling. The achieved stability of the ink compositions guaranteed their safe use within the timescale of several days and is displayed in the example in
Figure 2. The dissolved macromolecules of non-ionic surfactant in the dispersion medium avoid the flocculation of nanoparticles by means of steric stabilization through the functional groups having an affinity to the particle surface. The hydrophilic segments form a repulsive layer suppressing the coagulation of the nanoparticles present and stabilize them. On the role and effects of the dispersant agents in ink-jet inks, the reader is referred to Soleimani-Gorgani [
27] and Young-Sang Cho [
28] for further details.
The composition and processing properties of used inks with the inkjet printer DMP Dimatix 2800 Series can be analyzed rationally with the use of dimensionless criteria. Utilization of this analysis greatly enhanced the original trial-error development of the ink and accelerated optimization of the ink printing conditions. The reader is referred to the comprehensive works of Shlomo Magdassi [
16], Zhidong Pan [
29] and E. Kim and J. Baek [
30]. According to the literature, the following criteria are the most important. Generally, the printability range used to be determined by a
Z number, which is the inverse of the Ohnesorge number (
Oh). The recommendations for the optimum range of the Z number vary from 1–10 up to 4–14. However, it was found that the
Z number (or obviously
Oh number too) alone is insufficient for describing the droplet formation dynamics because all the terms describing dynamic effects are cancelled in its formula
and only the material constants and characteristic length remain [
30]. Actually, the characteristic length (
A) is a tool-related property since it is a printing nozzle square shaped orifice characteristic diameter, i.e., the side size of the square (
A = 21.5 µm). Therefore, other important non-dimensional parameters such as the Reynolds number (
Re), the Weber number (
We), and the capillary number (
Ca) should also be taken into consideration to complete the material-process-tool parameter-related triad.
Re represents the ratio between the viscous and the inertial forces in moving fluid,
We is dependent on the ratio between the inertia and the surface tension,
Oh reflects the physical properties of the liquid (the viscous forces, the inertial and the surface tension forces) and the size scale of the nozzle, but is independent of the driving conditions, and
Ca is the ratio of the viscous force to the capillary force.
Table 2 summarises the calculated dimensionless criteria of the prepared inks with the use of ink ejection fluid velocity finally estimated with the help of analysis discussed below. The influence of gravity is considered as being of very low relevance on the investigated range of parameters (including the stand-off being 1 mm) and therefore is not considered in this analysis. The formulas and description of used variables are given in
Appendix A—Used equations for dimensionless criteria.
The prepared suspensions were intentionally theoretically treated as Newtonian fluids only, neglecting the eventual weak viscoelasticity. Such an approach simplifies the problem radically; however, it can be applied until it produces predictions comparable with experiment. According to an analysis of ink-jet printing regimes by E. Kim and J. Baek [
30], all prepared inks fall into the “regime II” which is characterized by relatively small Ca and large We numbers. These conditions are manifested by a satellite formation and the merging of the satellite with the main droplet during its flight, thus fulfilling the definition of good printability when “a single drop is formed either directly without a second pinch-off or the satellite drop merges with the main drop within its travel distance less than 20 times A forming thus a single drop”. Referring back to literature sources, a simpler and earlier solution involving all material-tool-process characteristics may be found in the work of McKinley and Renardy [
31] who redrew the schematic diagram originally constructed by Derby [
32] to show the field of parameters for stable operations of drop on demand inkjet printing by using logarithmic coordinate system defined by plotting Ohnesorge against Reynolds number. The graph constructed with the help of their definition of printability boundaries is presented in
Figure 3. The quadrangle ABCD defines a region, in which the particular fluids are printable and single drop formation may be achieved or merging with the satellite can be expected. The diagram does not resolve between regime I and II as defined later by E. Kim and J. Baek [
27].
The purpose of printing process optimization is an achievement of the good printability conditions which is an absolute prerequisite for obtaining good-quality thin film layer including its precise location, resolution and functionality. We found it to be useful to discuss the printing parameters according to the suggestions of the printer producer and compare them with our experimental procedure. The small black full quadrangle in the center of the ABCD area in
Figure 3 represents the optimum printability space for the Dimatix printer (i.e., viscosity 10–12 mPa·s and the surface tension in the range of 28–42 mN·m
−1) while the typical value of the density of our suspensions varies slightly between 1.1 g∙mL
−1 and 1.3 g∙mL
−1, therefore 1.2 g∙mL
−1 was chosen for the model. The droplet velocity can be varied by the waveform process control; nonetheless our typical final optimum value 6 m∙s
−1 is taken into account in this analysis. Finally, the characteristic length
A is strictly given by the nozzle geometry in the used printing heads and cannot be changed at all. The changes of the variables involved in the dimensionless criteria result in typical shifts or extensions of this parameter space in the directions indicated by the six arrows marked from
a to
f. Decreasing the fluid velocity corresponds to the direction
a while the use of higher velocity shifts the area in the direction
d. Increasing the surface tension shifts the parameter space border downwards in direction
f while decreasing the surface tension results in the shift upwards in direction
c. Increasing the fluid viscosity results in the shift along the arrow
b and decreasing the viscosity extends the area in the direction of the arrow
e. Changes in the characteristic length or the density will result in a diagonal shift too, however, the slope will be −1/2. The full extension of the printability span according to the extreme viscosities and the surface tensions declared by the printer producer is indicated by the quadrangle with asterisks in its corners and the short dash dot sides. The position of the prepared inks is marked by the four full circle data points labelled by ITO concentrations. It must be noted that the points are aligned along a virtual line, which has slope −1 and represents a line with a constant value of
We1/2 of about 6.6 which corresponds to the average
We number value from
Table 2 of about 43.5. Although a good printability was finally achieved for all compositions by varying the parameters, the point closest to the centre of the printability area corresponds to the ink composition with 25 wt% of ITO.
Actually, the graph in
Figure 3 can be parametrised by a set of
We1/2 isolines based on the formula in Equation (1). These hyperbolic isolines are represented as straight lines using logarithmic axes. The similarity of these lines with the diagonal borders of the ABCD area invokes somewhat the old idea of the importance of a single dimensionless number again,
We number this case. The line AC corresponds exactly to the
We value 9 (means
We1/2 = 3). The importance of the
We criterion was raised by Derby himself again in [
33], where a corrected version of his original printability graph is published. However,
We value 4 is used by Derby according to Duineveld et al. [
34] as a minimum Weber number for a drop generation thus delineating the border to overcome the surface tension at the exposed nozzle. This value corresponds to the dotted line A’C’ with
We1/2 = 2 in the graph in
Figure 3, predicting a larger printability area. On the other hand, the splashing threshold line BD is not characterized by one value of the Weber number only. Actually,
We1/2 linearly varies from 20 for the point B to 13 for the point D. To generalize this lesson, we believe that the exclusiveness of
We among all the other dimensionless numbers used for describing the printability of inks it is due to the prime importance of the surface tension among all the discussed ink-jet material characteristics. The absolute condition
sine qua non of printing is the formation of the ink droplets and there is only one physical variable that is the source of the forces forming the droplet spherical shape, and it is the surface tension. It shall be mentioned that if the fluid is viscoelastic, the elasticity will contribute to the drop formation also. Moreover, the Weber number also contains the tool (
A) and the process (
ν) characteristics in contrast to the
Z number which is based on the material characteristics only. In our specific case, we experienced the good printability when decreasing the
We value below 47.
3.2. Annealing Temperature Optimization
Optimizing the annealing temperature is the key step in post-printing treatment of the manufactured device. Indeed, this important factor (i) influences grain size, densification or porosity of metal oxide film, which is closely related to the sensing efficiency [
8,
35,
36] furthermore it (ii) determines the resistance of the film and (iii) allows the removal of additives, which could cause interactions with the gas being sensed and thus influence the response of the sensory layer. Finally, the annealing temperature influences (iv) the choice of an appropriate substrate for the final device. Therefore, a thermogravimetric analysis of the prepared ink compositions has been accomplished to observe the temperature ranges corresponding to the evaporation of solvents and the vaporization of surface active polymer additives. A decomposition of the ink proceeded as shown in
Figure 4. In the first two steps low-molecular substances (water and ethylene glycol) were volatilized. Their complete evaporation was achieved below a temperature of 200 °C. The complete decomposition step of the present surfactant and the dispersant agents was observed in a range of 300 °C to 400 °C hence the lowest annealing temperature for printed films was set to 400 °C, and other samples were made at 500 °C and 600 °C for comparison too.
The morphology of original ITO particles as well as annealed films is shown in
Figure 5a in the figure depicts the ITO nanopowder as received. Presence of a wide size distribution can be observed. The biggest tetragonal bipyramides are of size below 100 nm but the vast majority of particles is significantly smaller and the powder materials generally corresponds to the specification provided by the supplier (<50 nm particle size). Treating deposited layers at temperatures of 400 °C, 500 °C and 600 °C leads to the formation of separated grains, which are in physical contact, see
Figure 5b–d respectively. Formation of short necks between individual grains was not confirmed and could be expected for higher temperatures. Thus, the sensing mechanism can be described using the “grains” or “grain boundary” model, where the Schottky interface between the grains, the height of potential barrier and therefore the dimension of the depletion region variation depends on the ambient atmosphere composition. The conductivity of this treated type of nanocrystalline metal oxide operates by grain boundary space charge (band bending) on inter-grain contact interfaces as shown in
Figure 6 where
Rgi represents the average intergrain resistance,
d—the grain diameter,
EF—the Fermi level,
EC—the conductive band [
35,
37].
Higher annealing temperatures increase the contact between the grains and can lead to the sintering of the grains in the agglomerates (>500 °C) [
38], which contributes to electric transport and increases conductivity. On the other hand, lower annealing temperatures allow for forming a more porous structure and the volume of the layer is more accessible to the detected gas, thus, the active surface is kept [
35,
37]. As can be seen in
Figure 7, the formation of a granular and porous structure is evident. A higher concentration of the charge due to the densification of the structure at a higher annealing temperature of 600 °C, and on the contrary a more porous structure in depth containing less neck connected grains at a lower annealing temperature of 400 °C was observed using scanning tunnelling microscopy combined with surface morphology and scanning electron microscopy of printed and heated films.
3.4. Gas Sensing Test
Based on the semiconductor properties of indium tin oxide, sensory behaviour is categorized as electrical and electrochemical receptor or/and transducer. Indium tin oxide, as a degenerate type semiconductor, due to present oxygen vacancies and by Sn doping [
7], reacts depending on ambient atmosphere composition by changing the electrical conductivity. The electrical conductivity is transferred as a signal of the electrical resistance variation of the deposited film upon the introduction of reducing or oxidizing gases. The signal of electrical resistance increases/decreases depending on the metal oxide nature as a function of the partial pressure of target gas. The measured electrical resistance is calculated as a sensor response ratio
SR expressed as a percentage as defined [
21]:
where
Rair represents the sensor resistance in air free of vapours atmosphere conditions and
Rgas resistance upon vapours ambience.
The mechanism responsible for the changes in the conductivity/resistivity of the layer observed in the presence of vapours can be attributed to predominating models: oxygen ionosorption and oxygen-vacancy model (reduction-reoxidation mechanism). The ionosorption mechanism of the metal oxide sensor response to the gases assumes a reaction of exposure gases with directly pre adsorbed O
2 species, from ambient oxygen, on the film surface. Oxygen molecules capture free excess electrons from the valence band of the oxide lattice structure and changed as O
2−. This process continues by formation of O
− and leads to decreasing the carrier concentration and causes a slight increase in the resistance of the thin film. The reaction is described as follows:
The exposed reducing gas reacts with adsorbed oxygen ions on the surface of the sensitive film presented as [
3,
19,
21]:
This interaction replenishes the concentration of electrons. Hence, the response of the sensing layer to the reducing gas exposure is manifested as a decrease of its resistance for n-type semiconductors.
In the case of the oxygen-vacancy model intrinsic defects such as oxygen vacancies on the surface of the metal oxide semiconductor act as electron donors and the surface conductivity is therefore controlled by a reduction and reoxidation of gaseous oxygen. The target gas removes oxygen from the surface side of the lattice and produces oxygen vacancies, which introduce electrons into the conduction band and therefore increases the electrical conductivity. In the absence of a target gas, the oxygen vacancies are filled by gaseous oxygen, and captures electrons resulting in a decrease in the electrical conductivity [
3].
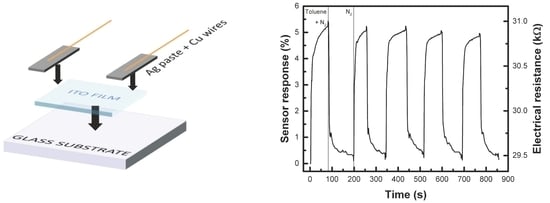
In accordance with the above-described mechanisms, we noticed a variation in the electrical resistance of the printed sensors (with a sensing layer prepared from ITO 25 wt% loading ink) upon exposure to saturated vapours of toluene in ambient air (toluene/air) at 25 °C as shown in
Figure 9 (upper graph window), which corresponds with the n-type semiconductor behaviour as described by Vaishnav [
19]. The response of the sensor operated in air can be explained by both the above-discussed mechanisms. However, the equilibrium in the reactions (3) and namely (4) is shifted to the left side at low temperatures (25–150 °C). The species O
− is believed to be dominant at an operating temperature of 300–450 °C while molecular forms should be favourable at low temperatures [
39,
40]. Therefore, we consider the oxygen vacancy model more plausible for operating the ITO sensor in air atmospheres. Baseline drift can be attributed to the presence of coexisting gases in an uncontrolled ambient laboratory atmosphere since we experienced it regardless to the pre-equlibration of the sensor. Because of the porous nature of the layer as well as a low operating temperature, the sensor requires a longer recovery time needed for the desorption and diffusion of vapour molecules from the porous film. We also observed that resistance increases when the sensor is exposed to saturated toluene vapours in an oxygen-free atmosphere as shown in
Figure 9 (lower graph window). This conductivity behaviour inversion can be attributed to a change to the sensing mechanism after placing the sensor into an N
2 atmosphere similarly as in [
3,
41,
42,
43]. However, none of the above-discussed models can apply for this case. It can be reasonably expected that oxygen adsorbates likely desorb from the surface in inert atmosphere, leaving behind electrons to the bulk of the semiconductor. On the other hand, the adsorption of electron accepting gas molecules causes a charge carrier transfer resulting in band bending and the formation of an electron-depleted region (so called space-charge layer), which is manifested as an increase in resistance. It is noteworthy that in a controlled inert atmosphere no baseline drift is experienced. The overall sensitivity of the prepared devices is in order of several % in both types of atmospheres (slightly higher in N
2) as measured at room temperature which can be explained by a high intrinsic conductivity of the ITO material and a suboptimal thickness of the sensing layer. The charge carrier density of ITO is reasonably high and the shape of the conduction band at the Fermi level faithfully retains the intrinsic parabolic character due to the contribution of Sn 5s electrons. This unique material property makes ITO a highly degenerate n-type semiconductor or, alternatively, a low-carrier-concentration metal [
44]. The Debye length of ITO is very small and therefore also the depletion layer on the particle surface is extremely thin hence the main contribution to the sensing layer conductivity is due to the grain volumes which is not affected by the stimulating gas adsorption/desorption [
40]. On the other hand, a fast response and recovery of the sensor resistance was experienced, and the performance (sensitivity) of the prepared device can be assessed as very promising if room temperature is considered as the operating temperature.