Magnetic Resonance Imaging Compatible Non-Invasive Fibre-Optic Sensors Based on the Bragg Gratings and Interferometers in the Application of Monitoring Heart and Respiration Rate of the Human Body: A Comparative Study
Abstract
:1. Introduction
2. State of the Art
3. Methods
3.1. Interferometric Phonocardiography
3.2. Grating Ballistocardiography
3.3. Non-Invasive Interferometric Measuring System
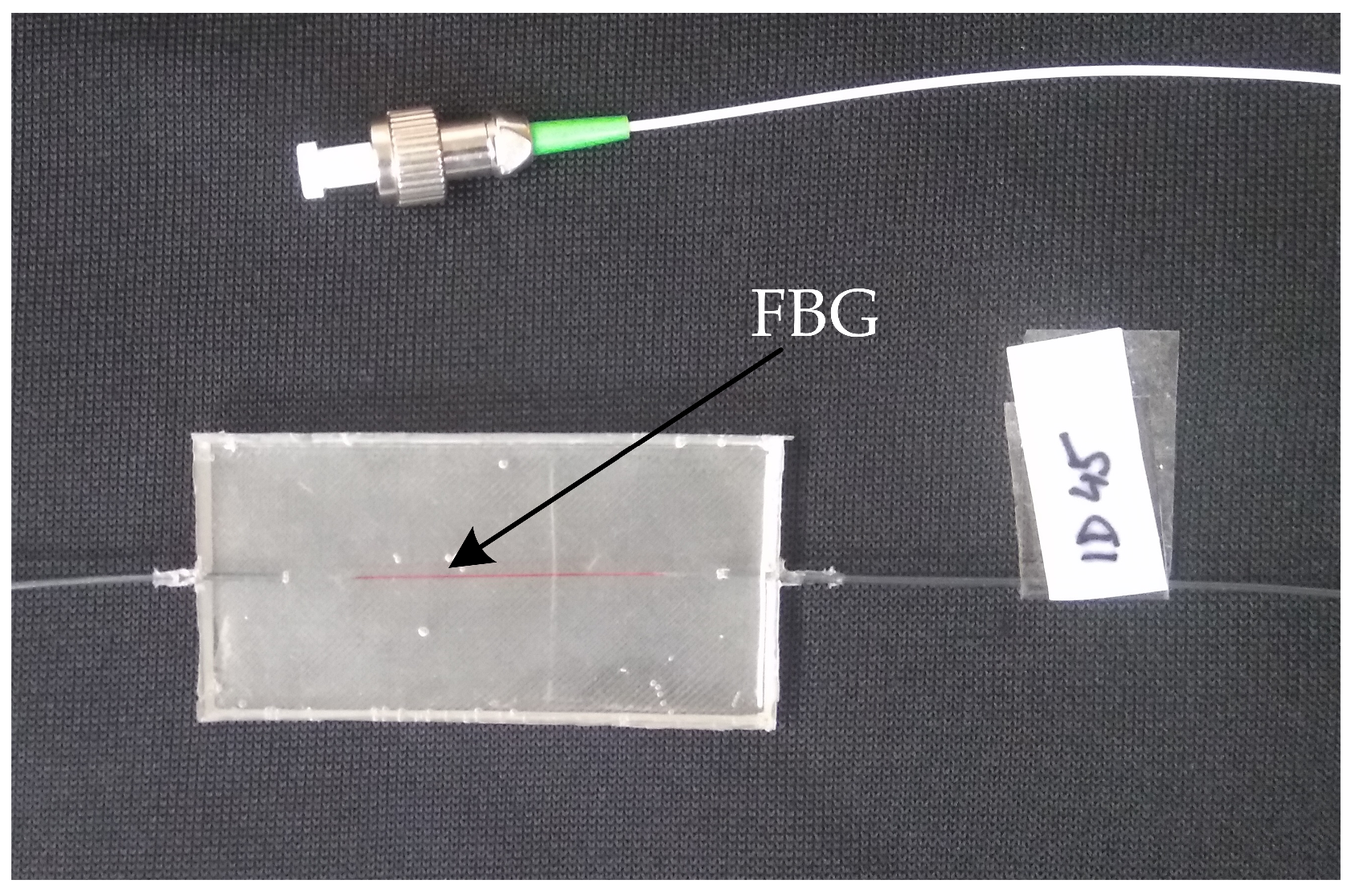
3.4. Non-Invasive FBG Measuring System
3.5. Reference Signals
3.6. Schematic Diagram of the Measurement
4. Results
4.1. The First Phase of the Comparative Study
4.2. Comparative Measurement of Vital Functions in Laboratory Conditions
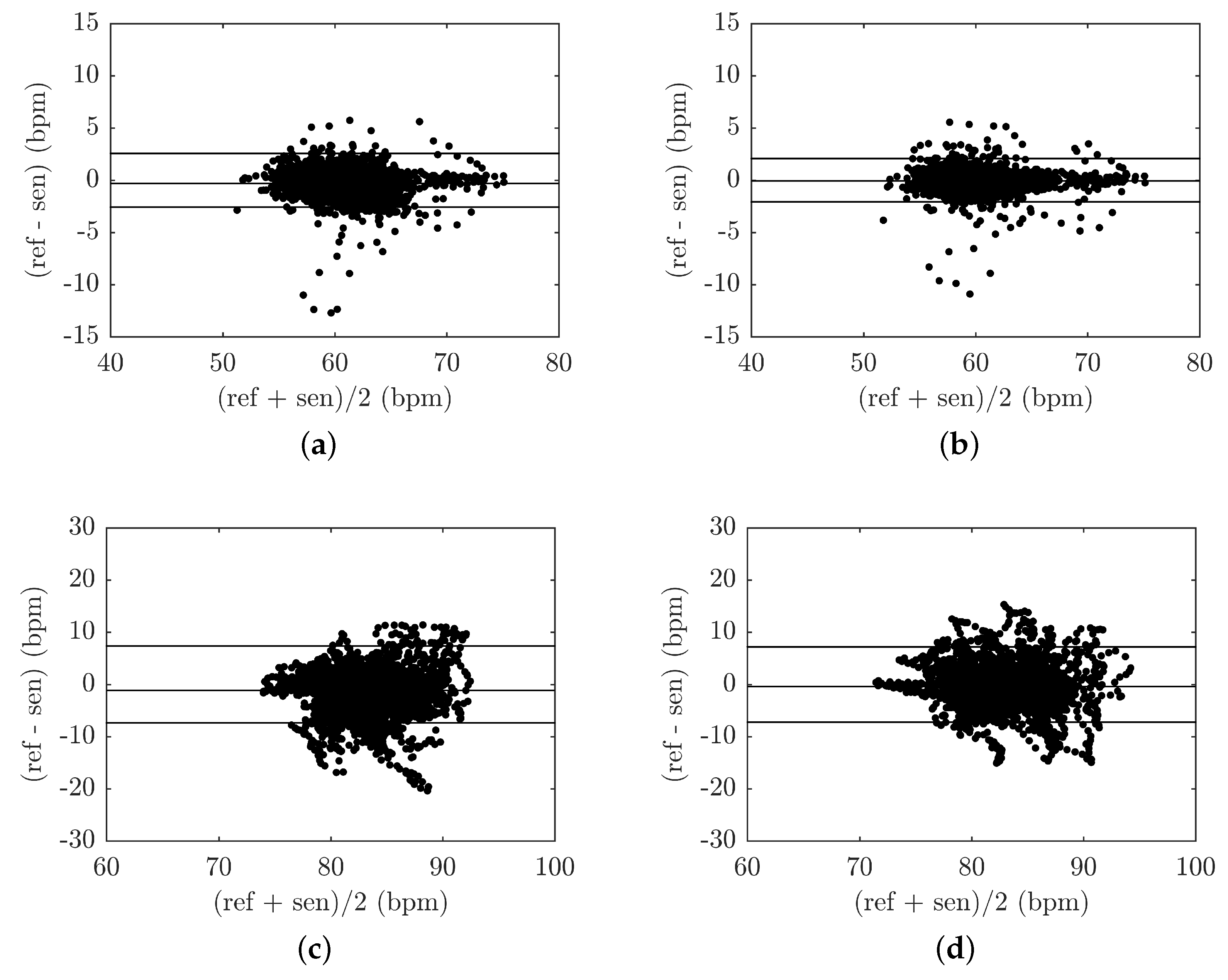
4.2.1. Respiratory Rate Measurement
4.2.2. Heart Rate Measurement
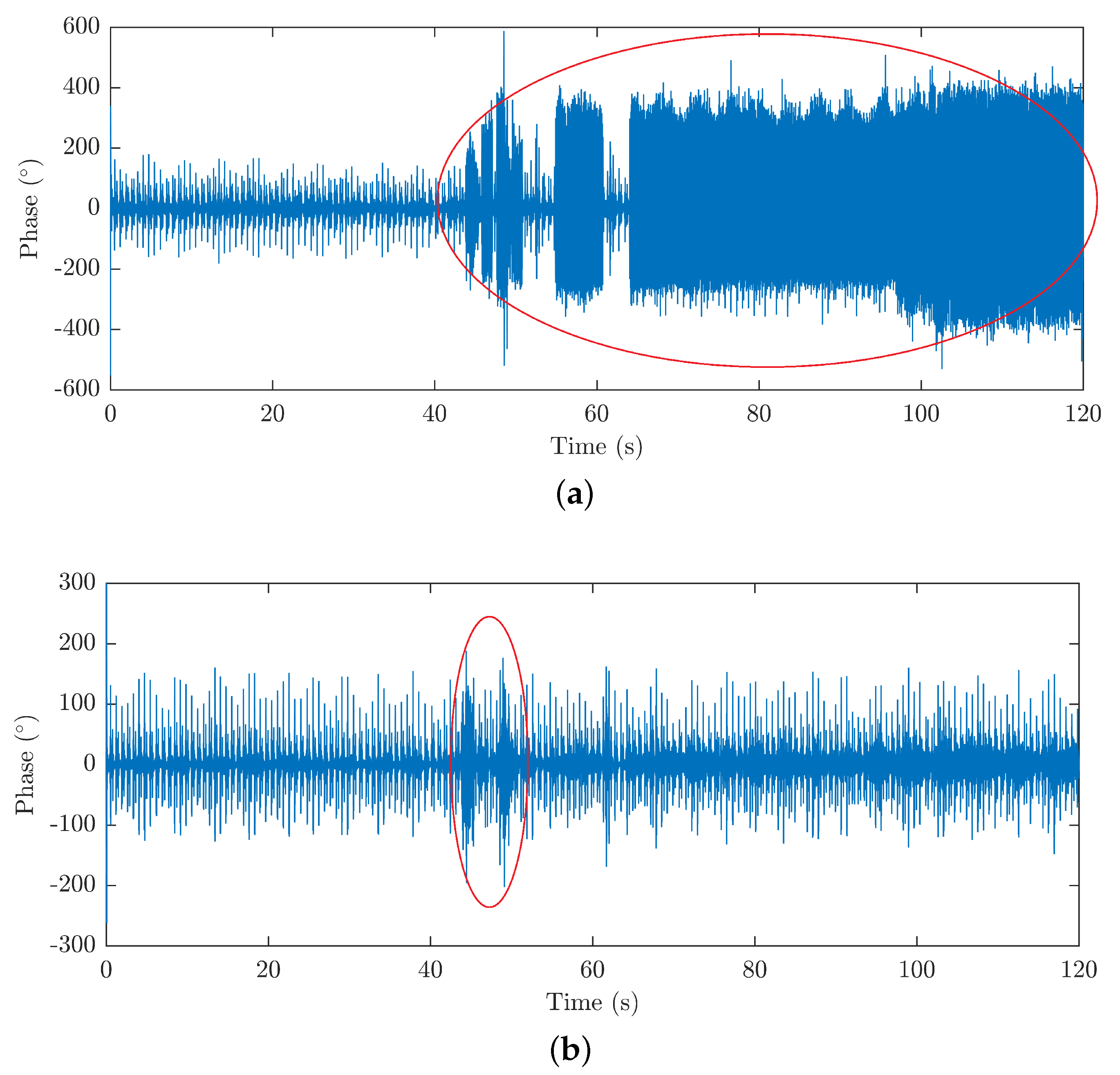
4.2.3. The Influence of Motion and Acoustic Artifacts on the HR and RR Signals Measured
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roriz, P.; Carvalho, L.; Frazão, O.; Santos, J.L.; Simões, A.J. From conventional sensors to fibre optic sensors for strain and force measurements in biomechanics applications: A review. J. Biomech. 2014, 47, 1251–1261. [Google Scholar] [CrossRef] [PubMed]
- Fendinger, N.J. Polydimethylsiloxane (PDMS): Environmental Fate and Effects. In Organosilicon Chemistry Set; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2005. [Google Scholar]
- Nedoma, J.; Fajkus, M.; Vasinek, V.; Bednarek, L.; Frnda, J.; Zavadil, J. Encapsulation of FBG sensor into the PDMS and its effect on spectral and power characteristics. Adv. Electr. Electron. Eng. 2016. [Google Scholar] [CrossRef]
- Nedoma, J.; Fajkus, M.; Siska, P.; Martinek, R.; Vasinek, V. Non-invasive fiber optic probe encapsulated into PolyDiMethylSiloxane for measuring respiratory and heart rate of the human body. Adv. Electr. Electr. Eng. 2017, 15, 93–100. [Google Scholar] [CrossRef]
- Martinek, R.; Nedoma, J.; Fajkus, M.; Kahankova, R.; Konecny, J.; Janku, P.; Kepak, S.; Bilik, P.; Nazeran, H. A Phonocardiographic-Based Fiber-Optic Sensor and Adaptive Filtering System for Noninvasive Continuous Fetal Heart Rate Monitoring. Sensors 2017, 17, 890. [Google Scholar] [CrossRef] [PubMed]
- Nedoma, J.; Fajkus, M.; Vasinek, V. Influence of PDMS encapsulation on the sensitivity and frequency range of fiber-optic interferometer. Proc. SPIE 2016. [Google Scholar] [CrossRef]
- Zboril, O.; Cubik, J.; Kepak, S.; Nedoma, J.; Fajkus, M.; Zavodny, P.; Vasinek, V. Noninvasive encapsulated fiber optic probes for interferometric measurement. Proc. SPIE Int. Soc. Opt. Eng. 2017. [Google Scholar] [CrossRef]
- Series, G. Transmission Systems and Media, Digital Systems and Networks. In Characteristics of a Single-Mode Optical Fibre and Cable-Recommendation ITU-T G.652; International Telecommunication Union: Geneva, Switzerland, 2016. [Google Scholar]
- Weckesser, M.; Posse, S.; Olthoff, U.; Kemna, L.; Dager, S.; Müller-Gärtner, H.W. Functional imaging of the visual cortex with bold-contrast MRI: Hyperventilation decreases signal response. Magn. Reson. Med. 1999, 41, 213–216. [Google Scholar] [CrossRef] [Green Version]
- Giardino, N.D.; Friedman, S.D.; Dager, S.R. Anxiety, Respiration and Cerebral Blood Flow: Implications for Functional Brain Imaging. Compr. Psychiatry 2007, 48, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Webster, J.G. Biomedical instrumentation. In Handbook of Research on Biomedical Engineering Education and Advanced Bioengineering Learning: Interdisciplinary Concepts; IGI Publishing: Hershey, PA, USA, 2012; pp. 339–355. [Google Scholar]
- Biel, L.; Pettersson, O.; Philipson, L.; Wide, P. ECG analysis: A new approach in human identification. IEEE Trans. Instrum. Meas. 2001, 50, 808–812. [Google Scholar] [CrossRef]
- Tamaki, S.; Yamada, T.; Okuyama, Y.; Morita, T.; Sanada, S.; Tsukamoto, Y.; Hori, M. Cardiac iodine-123 metaiodobenzylguanidine imaging predicts sudden cardiac death independently of left ventricular ejection fraction in patients with chronic heart failure and left ventricular systolic dysfunction: Results from a comparative study with signal-averaged electrocardiogram, heart rate variability, and QT dispersion. J. Am. Coll. Cardiol. 2009, 53, 426–435. [Google Scholar] [PubMed]
- Zabel, M.; Acar, B.; Klingenheben, T.; Franz, M.R.; Hohnloser, S.H.; Malik, M. Analysis of 12-lead T-wave morphology for risk stratification after myocardial infarction. Circulation 2000, 102, 1252–1257. [Google Scholar] [CrossRef] [PubMed]
- Ogura, R.; Hiasa, Y.; Takahashi, T.; Yamaguchi, K.; Fujiwara, K.; Ohara, Y.; Hosokawa, S. Specific findings of the standard 12-lead ECG in patients withtakotsubo’cardiomyopathy. Circ. J. 2003, 67, 687–690. [Google Scholar] [CrossRef] [PubMed]
- Hen, G.; Imtiaz, S.A.; Aguilar-Pelaez, E.; Rodriguez-Villegas, E. Algorithm for heart rate extraction in a novel wearable acoustic sensor. Healthc. Technol. Lett. 2015, 2, 28–33. [Google Scholar] [Green Version]
- Tóth, S.; Šajty, M.; Pekárová, T.; Mughees, A.; Štefanič, P.; Katz, M.; Spišáková, K.; Pella, J.; Pella, D. Addition of omega-3 fatty acid and coenzyme Q10 to statin therapy in patients with combined dyslipidemia. J. Basic Clin. Physiol. Pharmacol. 2017, 28, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Luna-Lozano, P.S.; García-Zetina, O.A.; Pérez-López, J.A.; Alvarado-Serrano, C. Portable device for heart rate monitoring based on impedance pletysmography. In Proceedings of the 2014 11th International Conference on Electrical Engineering, Computing Science and Automatic Control, CCE 2014, Campeche, Mexico, 29 September–3 October 2014. [Google Scholar]
- Burke, M.J.; Boilson, A. A digital cardiotachometer ASIC. In Proceedings of the Third International Conference on Electronics, Circuits, and Systems, Rodos, Greece, 16 October 1996; Volume 2, pp. 1080–1083. [Google Scholar]
- Pottinger, H.J.; Hughes, C.W.; Schroeder, P.; Barefield, A.; Craigmile, J.C. A microcomputer-based cardiotachometer with video display. Behav. Res. Methods Instrum. 1981, 13, 227–234. [Google Scholar] [CrossRef] [Green Version]
- Rotariu, C.; Cristea, C.; Arotaritei, D.; Bozomitu, R.G.; Pasarica, A. Continuous respiratory monitoring device for detection of sleep apnea episodes. In Proceedings of the 2016 IEEE 22nd International Symposium for Design and Technology in Electronic Packaging, SIITME 2016, Svasta, Paul, 20–23 October 2016; pp. 106–109. [Google Scholar]
- Yoon, J.-W.; Noh, Y.-S.; Kwon, Y.-S.; Kim, W.-K.; Yoon, H.-R. Improvement of dynamic respiration monitoring through sensor fusion of accelerometer and gyro-sensor. J. Electr. Eng. Technol. 2014, 9, 334–343. [Google Scholar] [CrossRef]
- Byung-Hyun, K.; Jong-Gwan, Y. Human health monitoring technology. In Proceedings of the Micro- and Nanotechnology Sensors, Systems, and Applications IX, Anaheim, CA, USA, 9–13 April 2017. [Google Scholar]
- Will, C.; Shi, K.; Lurz, F.; Weigel, R.; Koelpin, A. Intelligent signal processing routine for instantaneous heart rate detection using a Six-Port microwave interferometer. In Proceedings of the 2015 International Symposium on Intelligent Signal Processing and Communication Systems (ISPACS), Nusa Dua, Bali, 9–12 November 2015; pp. 483–487. [Google Scholar]
- Jourand, P.; De Clercq, H.; Puers, R. Robust monitoring of vital signs integrated in textile. Sens. Actuators A Phys. 2010, 161, 288–296. [Google Scholar] [CrossRef]
- Hong, S.; Jung, W.; Kim, T.; Oh, K. Compact Biocompatible Fiber Optic Temperature Microprobe Using DNA-Based Biopolymer. J. Lightw. Technol. 2018, 36, 974–978. [Google Scholar] [CrossRef]
- Podbreznik, P.; Đonlagić, D.; Lešnik, D.; Cigale, B.; Zazula, D. Nemoteče spremljanje delovanja srca z optičnim senzorjem v postelji Unobtrusive heartbeat monitoring by using a bed fiber-optic sensor. Zdravniski Vestnik 2014, 83, 901–907. [Google Scholar]
- Podbreznik, P.; Onlagić, D.; Lešnik, D.; Cigale, B.; Zazula, D. Cost-efficient speckle interferometry with plastic optical fiber for unobtrusive monitoring of human vital signs. J. Biomed. Opt. 2013, 18. [Google Scholar] [CrossRef] [PubMed]
- Favero, F.C.; Villatoro, J.; Pruneri, V. Microstructured optical fiber interferometric breathing sensor. J. Biomed. Opt. 2012. [Google Scholar] [CrossRef] [PubMed]
- Sprager, S.; Donlagic, D.; Zazula, D. Monitoring of basic human vital functions using optical interferometer. In Proceedings of the IEEE 10th International Conference on Signal Processing (ICSP), Beijing, China, 24–28 October 2010; pp. 1738–1741. [Google Scholar]
- Folke, M.; Cernerud, L.; Ekstrom, M.; Hok, B. Critical review of non-invasive respiratory monitoring in medical care. Med. Biol. Eng. Comput. 2003, 41, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Sprager, S.; Donlagic, D.; Zazula, D. Estimation of heart rate, respiratory rate and motion by using optical interferometer as body sensor. In Proceedings of the IASTED International Conference on Signal and Image Processing, Honolulu, HI, USA, 14–16 December 2011; pp. 280–287. [Google Scholar]
- Sprager, S.; Holobar, A.; Zazula, D. Feasibility Study of Heartbeat Detection from Optical Interferometric Signal by Using Convolution Kernel Compensation. In Proceedings of the 6th International Conference on Bio-inspired Systems and Signal Processing, Barcelona, Spain, 11–14 February 2013. [Google Scholar]
- Šprager, S.; Zazula, D. Detection of heartbeat and respiration from optical interferometric signal by using wavelet transform. Comput. Methods Prog. Biomed. 2013, 111, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Cikajlo, I.; Sprager, S.; Erjavec, T.; Zazula, D. Cardiac arrhythmia alarm from optical interferometric signals during resting or sleeping for early intervention. Biocybern. Biomed. Eng. 2016, 36, 267–275. [Google Scholar] [CrossRef]
- Zazula, D.; Sprager, S. Detection of the first heart sound using fibre-optic interferometric measurements and neural networks. In Proceedings of the Symposium on Neural Network Applications in Electrical Engineering, Belgrade, Serbia, 20–22 September 2012; pp. 171–176. [Google Scholar]
- Byeong, H.L.; Young, H.K.; Kwan, S.P.; Joo, B.E.; Myoung, J.K.; Byung, S.R.; Hae, Y.C. Interferometric Fiber Optic Sensors. Sensors 2012, 12, 2467–2486. [Google Scholar] [Green Version]
- Hsieh, Y.H.; Chen, N.K. Micro tapered Mach–Zehnder fiber interferometer for monitoring pressure fluctuation and its applications in pulse rate detection. In Proceedings of the 2013 6th IEEE/International Conference on Advanced Infocomm Technology (ICAIT), Taiwan, China, 6–9 July 2013; pp. 113–115. [Google Scholar]
- Chethana, K.; Guru Prasad, A.S.; Omkar, S.N.; Asokan, S. Fiber bragg grating sensor-based device for simultaneous measurement of respiratory and cardiac activities. J. Biophotonics 2016, 10, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, M.; Sonobe, M.; Watanabe, K. Unconstrained pulse pressure monitoring for health management using hetero-core fiber optic sensor. Biomed. Opt. Express 2016, 7, 3675–3685. [Google Scholar] [CrossRef] [PubMed]
- Dziuda, L. Fiber-optic sensors for monitoring patient physiological parameters: A review of applicable technics and relevance to use during MRI procedures. J. Biomech. 2015, 20. [Google Scholar] [CrossRef]
- Gurkan, D.; Starodubov, D.; Yuan, X. Monitoring of the heartbeat sounds using an optical fiber Bragg grating sensor. IEEE Sens. 2005, 2005, 306–309. [Google Scholar]
- Yang, X.; Chen, Z.; Elvin, C.S.M.; Janice, L.H.Y.; Ng, S.H.; Teo, J.T.; Wu, R. Textile Fiber Optic Microbend Sensor Used for Heartbeat and Respiration Monitoring. IEEE Sens. J. 2015, 15, 757–761. [Google Scholar] [CrossRef]
- Ciocchetti, M.; Massaroni, C.; Saccomandi, P.; Caponero, M.A.; Polimadei, A.; Formica, D.; Schena, E. Smart textile based on fiber bragg grating sensors for respiratory monitoring: Design and preliminary trials. Biosensors 2015, 5, 602–615. [Google Scholar] [CrossRef] [PubMed]
- Dziuda, L.; Krej, M.; Skibniewski, F.W. Fiber Bragg grating strain sensor incorporated to monitor patient vital signs during MRI. IEEE Sens. J. 2013, 13, 4986–4991. [Google Scholar] [CrossRef]
- Dziuda, L.; Skibniewski, F.W.; Krej, M.; Baran, P.M. Fiber Bragg grating-based sensor for monitoring respiration and heart activity during magnetic resonance imaging examinations. J. Biomed. Opt. 2013, 18, 057006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Teo, J.T.; Ng, S.H.; Yim, H. Smart pillow for heart rate monitoring using a fiber optic sensor. In Proceedings of the SPIE BiOS, San Francisco, CA, USA, 22–27 January 2011. [Google Scholar]
- Chen, Z.H.; Teo, J.T.; Yang, X.F. In-bed fibre optic breathing and movement sensor for non-intrusive monitoring. In Proceedings of the SPIE BiOS, San Jose, CA, USA, 24–29 January 2009. [Google Scholar]
- Dzuida, L.; Skibniewski, F.W.; Krej, M.; Lewandowski, J. Monitoring respiration and cardiac activity using fiber Bragg grating-based sensor. IEEE Trans. Biomed. Eng. 2012, 59, 1934–1942. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Koyama, S.; Ishizawa, H.; Fujiwara, S.; Fujimoto, K. Simultaneous Measurement of Heart Sound, Pulse Wave and Respiration with Single Fiber Bragg Grating Sensor. In Proceedings of the MeMeA 2018—2018 IEEE International Symposium on Medical Measurements and Applications, Rome, Italy, 11–13 June 2018. [Google Scholar] [CrossRef]
- Lau, D.; Chen, Z.; Teo, J.T.; Ng, S.H.; Rumpel, H.; Lian, Y.; Yang, H.; Kei, P.L. Intensity-modulated microbend fiber optic sensor for respiratory monitoring and gating during MRI. IEEE Trans. Biomed. Eng. 2013, 60, 2655–2662. [Google Scholar] [CrossRef] [PubMed]
- Grilett, A.; Kinet, D.; Witt, J.; Schukar, M.; Krebber, K.; Pirotte, F.; Depre, A. Optical fiber sensors embedded into medical textiles for healthcare monitoring. IEEE Sens. 2008, 2008, 1215–1222. [Google Scholar] [CrossRef]
- Mokhtar, M.R.; Sun, T.; Grattan, K.T.V. Bragg Grating Packages with Nonuniform Dimensions for Strain and Temperature Sensing. IEEE Sens. J. 2012, 12, 139–144. [Google Scholar] [CrossRef]
- Tosi, D.; Olivero, M.; Perrone, G. Low-cost fiber bragg grating vibroacoustic sensor for voice and heartbeat detection. Appl. Opt. 2008, 47, 5123–5129. [Google Scholar] [CrossRef] [PubMed]
- Wo, J.; Wang, H.; Sun, Q.; Shum, P.P.; Liu, D. Noninvasive respiration movement sensor based on distributed Bragg reflector fiber laser with beat frequency interrogation. J. Biomed. Opt. 2014, 19. [Google Scholar] [CrossRef] [PubMed]
- Dziuda, L.; Skibniewski, F.W. A new approach to ballistocardiographic measurements using fibre Bragg grating-based sensors. Biocybern. Biomed. Eng. 2014, 34, 101–106. [Google Scholar] [CrossRef]
- Spillman, W.B.; Mayer, M.; Bennett, J.; Gong, J.; Meissner, K.E.; Davis, B.; Xu, X. A ’smart’ bed for non-intrusive monitoring of patient physiological factors. J. Meas. Sci. Technol. 2004, 15, 1614–1620. [Google Scholar] [CrossRef]
- Witt, J.; Narbonneau, F.; Schukar, M.; Krebber, K.; De Jonckheere, J.; Jeanne, M.; Thiel, T. Medical textiles with embedded fiber optic sensors for monitoring of respiratory movement. IEEE Sens. J. 2012, 12, 246–254. [Google Scholar] [CrossRef]
- Fajkus, M.; Nedoma, J.; Martinek, R.; Vasinek, V.; Nazeran, H.; Siska, P. A Non-invasive Multichannel Hybrid Fiber-optic Sensor System for Vital Sign Monitoring. Sensors 2017, 17, 111. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Lau, D.; Teo, J.T.; Ng, S.H.; Yang, X.; Kei, P.L. Simultaneous measurement of breathing rate and heart rate using a microbend multimode fiber optic sensor. J. Biomed. Opt. 2014, 19. [Google Scholar] [CrossRef] [PubMed]
- Deepu, C.J.; Chen, Z.; Teo, J.T.; Ng, S.H.; Yang, X.; Lian, Y. A smart cushion for real-time heart rate monitoring. In Proceedings of the 2012 IEEE Biomedical Circuits and Systems Conference (BioCAS), Taiwan, China, 28–30 November 2012; pp. 53–56. [Google Scholar]
- Lydtin, H.; Schnelle, K.; Lohmöller, G.; Zöllner, N. Synchronous recordings of Bcg, ECG, carotid pulse wave, and PCG in acute pharmacological trials. Bibl. Cardiol. 1969, 26, 36–41. [Google Scholar] [PubMed]
- Martín-Yebra, A.; Landreani, F.; Casellato, C.; Pavan, E.; Frigo, C.; Migeotte, P.-F.; Caiani, E.G. Studying heart rate variability from ballistocardiography acquired by force platform: Comparison with conventional ECG. In Proceedings of the 42nd Conference of Computing in Cardiology, Nice, France, 6–9 September 2015; pp. 929–932. [Google Scholar]
- Al-Qazzaz, N.K.; Abdulazez, I.F.; Ridha, S.A. Simulation Recording of an ECG, PCG, and PPG for Feature Extractions. Al-Khwarizmi Eng. J. 2014, 10, 81–91. [Google Scholar]
- Varshneya, D.; Maida, J.L. Fiber-Optic Monitor Using Interferometry for Detecting Vital Signs of a Patient. U.S. Patent No. 6498652, 24 December 2002. [Google Scholar]
- Varshneya, D.; Maida, J.L.; Jeffers, L.A. Fiber-Optic Interferometric Vital Sign Monitor for Use in Magnetic Resonance Imaging, Confined Care Facilities and in-Hospital. U.S. Patent No. 6816266, 9 November 2004. [Google Scholar]
- Zyczkowski, M.; Uzieblo-Zyczkowska, B. Human psychophysiology activity monitoring methods using fiber optic sensors. In Proceedings of the International Society for Optical Engineering, San Diego, CA, USA, 2 August 2010. [Google Scholar]
- Nedoma, J.; Fajkus, M.; Kepak, S.; Cubik, J.; Kahankova, R.; Janku, P.; Vasinek, V.; Nazeran, H.; Martinek, R. Noninvasive fetal heart rate monitoring: Validation of phonocardiography-based fiber-optic sensing and adaptive filtering using the nlms algorithm. Adv. Electr. Electron. Eng. 2017, 15, 544–552. [Google Scholar] [CrossRef]
- Zazula, D.; Đonlagic, D.; Sprager, S. Application of fiber-optic interferometry to detection of human vital signs. J. Laser Health Acad. 2012, 2012, 27–32. [Google Scholar]
- Chen, Q.; Claus, R.O.; Spillman, W.B.; Arregui, F.J.; Matias, I.R.; Cooper, K.L. Optical fiber sensors for breathing diagnostics. In Proceedings of the Optical Fiber Sensors Conference Technical Digest, Portland, OR, USA, 6–11 May 2002. [Google Scholar]
- Kang, Y.; Ruan, H.; Wang, Y.; Arregui, F.J.; Matias, I.R.; Claus, R.O. Nanostructured optical fiber sensors for breathing airflow monitoring. Meas. Sci. Technol. 2006, 17, 1207–1210. [Google Scholar] [CrossRef]
- Kang, Y.; Ruan, H.; Mecham, J.; Wang, Y.; Arregui, F.J.; Matias, I.R.; Claus, R.O. Nanostructured optical fiber sensors for breathing airflow monitoring. Proc. SPIE Int. Soc. Opt. Eng. 2005, 407–410. [Google Scholar] [CrossRef]
- Durand, L.G.; Pibarot, P. Digital signal processing of the phonocardiogram: Review of the most recent advancements. Crit. Rev. Biomed. Eng. 1995, 23, 3–4. [Google Scholar] [CrossRef]
- Webster, J.G. Medical Devices and Instrumentation; Wiley-Interscience: Hoboken, NJ, USA, 1988. [Google Scholar]
- Phanphaisarn, W.; Roeksabutr, A.; Wardkein, P.; Koseeyaporn, J.; Yupapin, P.P. Heart detection and diagnosis based on Ecg and EPcg relationships. Med. Dev. 2011, 4. [Google Scholar] [CrossRef]
- Southern, E.M. Electrocardiography and phonocardiography of the foetal heart. Int. J. Obstet. Gynaecol. 1954, 61, 231–237. [Google Scholar] [CrossRef]
- Kersay, A.D.; Davis, M.A.; Patrick, H.J.; LeBlanc, M.; Koo, K.P.; Askins, C.G.; Putnam, M.A.; Friebele, E.J. Tunable Erbium-Doped Fiber Lasers Using Various Inline Fiber Filters. Engineering 2010, 2, 1442–1463. [Google Scholar]
- Pinheiro, E.; Postolache, O.; Girão, P. Theory and developments in an unobtrusive cardiovascular system representation: Ballistocardiography. Open Biomed. Eng. J. 2010, 4, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.W. Certain Molar Movements of the Human Body produced by the Circulation of the Blood. J. Anat. Physiol. 1877, 11, 533–536. [Google Scholar] [PubMed]
- Kepak, S.; Cubik, J.; Siska, P.; Zboril, O.; Martinek, R. A Device for Monitoring Vital Functions of a Pregnant Woman’s Foetus. Available online: https://isdv.upv.cz/webapp/webapp.pts.det?xprim=10232644&lan=cs&s_majs=&s_puvo=kep%C3%A1k%20stanislav&s_naze=&s_anot= (accessed on 30 October 2018).
- Chen, D.-S.; Xiao, L.; Cui, J.; Liu, T.-T.; Sun, D.-X. Analysis of 3 × 3 coupler demodulation method for optical fiber interferometer and polarization fading. J. Optoelectron. Laser 2007, 18, 523–525. [Google Scholar]
- Mao, X.; Huang, J.-B.; Gu, H.-C. Demodulation Technology of Distributed Feedback Fiber Laser Sensor Based on 3 × 3 Coupler. Chin. J. Lumin. 2017, 38, 395–401. [Google Scholar]
- Sprager, S.; Zazula, D. Heartbeat and respiration detection from optical interferometric signals by using a multimethod approach. IEEE Trans. Biomed. Eng. 2012, 59, 2922–2929. [Google Scholar] [CrossRef] [PubMed]
- UFI PnuemoTrace II Piezo-Electric Respiration Transducer. Available online: https://www.artisantg.com/Scientific/730671/UFI_PneumoTrace_II_1132_Piezo_Electric_Respiration_Transducer (accessed on 26 October 2018).
- Products. Available online: https://www.physio-control.com/ProductDetails.aspx (accessed on 26 October 2018).
- Taffoni, F.; Formica, D.; Saccomandi, P.; Di Pino, G.; Schena, E. Optical fiber-based MR-compatible sensors for medical applications: An overview. Sensors 2013, 13, 14105–14120. [Google Scholar] [CrossRef] [PubMed]
- Grillet, A.; Kinet, D.; Witt, J.; Schukar, M.; Krebber, K.; Pirotte, F.; Depré, A. Optical fibre sensors embedded into medical textiles for monitoring of respiratory movements in MRI environment. In Proceedings of the SPIE—The International Society for Optical Engineering, Napoli, Italy, 4 July 2007. [Google Scholar]
- Moerman, K.M.; Sprengers, A.M.J.; Nederveen, A.J.; Simms, C.K. A novel MRI compatible soft tissue indentor and fibre Bragg grating force sensor. Med. Eng. Phys. 2013, 35, 486–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, U.-X.; Yang, B.; Gullapalli, R.; Desai, J.P. Triaxial MRI-compatible fiber-optic force sensor. IEEE Trans. Robot. 2011, 27, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Yoo, W.J.; Jang, K.W.; Seo, J.K.; Heo, J.Y.; Moon, J.S.; Park, J.Y.; Lee, B. Development of respiration sensors using plastic optical fiber for respiratory monitoring inside MRI system. J. Opt. Soc. Korea 2010, 14, 235–239. [Google Scholar] [CrossRef]
- Kam, W.; Mohammed, W.S.; Leen, G.; O’Sullivan, K.; O’Keeffe, M.; O’Keeffe, S.; Lewis, E. All plastic optical fiber-based respiration monitoring sensor. In Proceedings of the IEEE Sensors, New Delhi, India, 29–31 December 2017; pp. 1–3. [Google Scholar]
- Filograno, M.L.; Pisco, M.; Catalano, A.; Forte, E.; Aiello, M.; Cavaliere, C.; Soricelli, A.; Davino, D.; Visone, C.; Cutolo, A.; et al. Triaxial Fiber Optic Magnetic Field Sensor for Magnetic Resonance Imaging. J. Lightw. Technol. 2017, 35, 3924–3933. [Google Scholar] [CrossRef]
- Nedoma, J.; Fajkus, M.; Novak, M.; Strbikova, N.; Vasinek, V.; Nazeran, H.; Vanus, J.; Perecar, F.; Martinek, R. Validation of a novel fiber-optic sensor system for monitoring cardiorespiratory activities during mri examinations. Adv. Electr. Electron. Eng. 2017, 15, 536–543. [Google Scholar] [CrossRef]
- Su, H.; Shang, W.; Li, G.; Patel, N.; Fischer, G.S. An MRI-Guided Telesurgery System Using a Fabry–Perot Interferometry Force Sensor and a Pneumatic Haptic Device. Ann. Biomed. Eng. 2017, 45, 1917–1928. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D.G. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 1999, 8, 135–160. [Google Scholar] [CrossRef] [PubMed]
- Signa HDxt 1.5T. Available online: http://www3.gehealthcare.in/en/products/categories/magnetic-resonance-imaging/signa-hdxt-1-5t (accessed on 26 October 2018).
- Counter, S.A.; Olofsson, A.; Grahn, H.F.; Borg, E. MRI acoustic noise: Sound pressure and frequency analysis. J. Mag. Reson. Imag. 1997, 7, 606–611. [Google Scholar] [CrossRef]
- Quandt, B.M.; Scherer, L.J.; Boesel, L.F.; Wolf, M.; Bona, G.-L.; Rossi, R.M. Body-Monitoring and Health Supervision by Means of Optical Fiber-Based Sensing Systems in Medical Textiles. Adv. Healthc. Mater. 2015, 4, 330–355. [Google Scholar] [CrossRef] [PubMed]














| Advantages | Disadvantages | Application Possibilities | Cost (Approximately) Probe/Evaluation Unit | |
|---|---|---|---|---|
| FBG system | the feed fibre is not sensitive to vibrations and other undesirable effects; one lead-in fibre for sensor; design of the sensor (probe) is extremely compact = small size; the possibility of quasi-distributed measurement - sensors can be multiplexed on a single optical fibre with single evaluation unit; the possibility of remote evaluation (hundreds meters); MRI-compatible | costly spectral evaluation (higher cost); low signal-to-noise ratio (in the case of multiple probes) | RR and HR of the human body | 100 $/18,000 $ |
| FOI system | higher sensitivity; measuring system can thus be constructed from commonly available components (low-cost); the potential for detecting acoustic signals (monitoring respiratory and arousal events); MRI-compatible | sensor’s dimensions (potentially discomfort); storing of the reference arm; a higher number of lead-in fibres for each sensor | RR and HR of the human body; monitoring respiratory and arousal events | 200 $/4000 $ |
| Interferometric Sensors | ||
| Reference | Type of Interferometer, Measured Parameter | Quantitative Data on Sensor Efficiency |
| [65,66] (Patents) | Mach–Zehnder, Michelson, Sagnac, Fabry–Perot (RR and HR) | No efficiency data presented by authors |
| [37,67,68] | Mach–Zehnder, Michelson, Sagnac, Fabry–Perot (RR and HR) | Insufficient data, our previously research: No efficiency data presented by authors |
| [33,34,35,36,69] | Michelson (RR and HR) | Most recent, experimental study: recording time: 60 s; sensitivity: 97.64 ± 7.28%, precision: 99.38 ± 2.80%, maximum relative error: 7.35 ± 7.20% for RR detection, sensitivity: 99.46 ± 1.11%, precision: 99.60 ± 1.05%, maximum relative error: 3.16 ± 2.32% for HR detection |
| [27] | Speckle interferometer (RR) | Sensitivity: 98.4 ± 1.1%, precision: 98.2 ± 2% |
| [38] | Micro tapered Mach–Zehnder (RR) | Insufficient data |
| [70,71,72] | Fabry–Perot interferometer (RR) | No efficiency data presented by authors |
| Fibre Bragg Grating Sensors | ||
| Reference | Measured Parameter | Quantitative Data on Sensor Efficiency |
| [39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61] | RR and/or HR | Most recent experimental study: An RMS (root mean square) value of the relative error is below 1.8%, our previously research (2 FBGs): maximum relative error 5.41% for RR detection, sensitivity 96.54% for HR detection |
| FBG Probe | Interferometric Probe | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sub. | Time (s) | ARR (rpm) | NoS (-) | Error (-) | Rel. Error (%) | Samples in ±1.96 SD (%) | NoS (-) | Error (-) | Rel. Error (%) | Samples in ±1.96 SD (%) |
| M1 | 3594 | 17 | 1025 | 45 | 4.39 | 95.61 | 1026 | 45 | 4.39 | 95.61 |
| M2 | 3557 | 16 | 957 | 41 | 4.28 | 95.72 | 957 | 40 | 4.18 | 95.82 |
| M3 | 3567 | 18 | 1057 | 39 | 3.69 | 96.31 | 1057 | 38 | 3.60 | 96.40 |
| M4 | 3612 | 17 | 1031 | 38 | 3.69 | 96.31 | 1031 | 37 | 3.59 | 96.41 |
| M5 | 3512 | 15 | 869 | 41 | 4.72 | 95.28 | 870 | 40 | 4.60 | 95.40 |
| M6 | 3517 | 16 | 939 | 37 | 3.94 | 96.06 | 939 | 37 | 3.94 | 96.06 |
| M7 | 3587 | 15 | 898 | 35 | 3.90 | 96.10 | 899 | 35 | 3.89 | 96.11 |
| M8 | 3574 | 17 | 1014 | 41 | 4.04 | 95.96 | 1015 | 40 | 3.94 | 96.06 |
| M9 | 3547 | 15 | 889 | 36 | 4.05 | 95.95 | 889 | 36 | 4.05 | 95.95 |
| M10 | 3568 | 18 | 1069 | 42 | 3.93 | 96.07 | 1071 | 42 | 3.92 | 96.0 |
| F1 | 3727 | 15 | 922 | 59 | 6.40 | 93.60 | 923 | 51 | 5.53 | 94.47 |
| F2 | 3624 | 11 | 667 | 37 | 5.55 | 94.45 | 667 | 35 | 5.25 | 94.75 |
| F3 | 3498 | 13 | 761 | 38 | 4.99 | 95.01 | 762 | 36 | 4.72 | 95.28 |
| F4 | 3614 | 12 | 719 | 34 | 4.73 | 95.27 | 719 | 34 | 4.73 | 95.27 |
| F5 | 3608 | 15 | 904 | 39 | 4.31 | 95.69 | 904 | 39 | 4.31 | 95.69 |
| F6 | 3578 | 14 | 835 | 33 | 3.95 | 96.05 | 836 | 31 | 3.71 | 96.29 |
| F7 | 3595 | 14 | 841 | 37 | 4.40 | 95.60 | 842 | 37 | 4.39 | 95.61 |
| F8 | 3587 | 15 | 898 | 42 | 4.68 | 95.32 | 898 | 41 | 4.57 | 95.43 |
| F9 | 3574 | 16 | 954 | 45 | 4.72 | 95.28 | 955 | 43 | 4.50 | 95.50 |
| F10 | 3604 | 14 | 842 | 43 | 5.11 | 94.89 | 842 | 42 | 4.99 | 95.01 |
| Sum | 71,644 | 18,091 | 802 | 4.47 | 95.53 | 18102 | 779 | 4.34 | 95.66 | |
| FBG Probe | FOI Probe | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subject | Time (s) | AHR (bpm) | NoS (-) | Error (-) | Rel. Error (%) | Samples in ±1.96 SD (%) | NoS (-) | Error (-) | Rel. Error (%) | Samples in ±1.96 SD (%) |
| M1 | 3602 | 63 | 3781 | 171 | 4.52 | 95.48 | 3781 | 121 | 3.20 | 96.80 |
| M2 | 3547 | 76 | 4487 | 197 | 4.39 | 95.61 | 4489 | 165 | 3.68 | 96.32 |
| M3 | 3628 | 77 | 4664 | 208 | 4.46 | 95.54 | 4665 | 158 | 3.39 | 96.61 |
| M4 | 3571 | 62 | 3691 | 185 | 5.01 | 94.99 | 3692 | 133 | 3.60 | 96.40 |
| M5 | 3498 | 76 | 4422 | 188 | 4.25 | 95.75 | 4422 | 159 | 3.60 | 96.40 |
| M6 | 3521 | 75 | 4398 | 185 | 4.21 | 95.79 | 4399 | 157 | 3.57 | 96.43 |
| M7 | 3617 | 71 | 4286 | 169 | 3.94 | 96.06 | 4288 | 149 | 3.47 | 96.53 |
| M8 | 3647 | 68 | 4128 | 168 | 4.07 | 95.93 | 4131 | 152 | 3.68 | 96.32 |
| M9 | 3645 | 66 | 4028 | 171 | 4.25 | 95.75 | 4029 | 147 | 3.65 | 96.35 |
| M10 | 3589 | 73 | 4382 | 185 | 4.22 | 95.78 | 4384 | 158 | 3.60 | 96.40 |
| F1 | 3507 | 82 | 4789 | 281 | 5.87 | 94.13 | 4781 | 187 | 3.91 | 96.09 |
| F2 | 3661 | 81 | 4941 | 266 | 5.38 | 94.62 | 4943 | 189 | 3.82 | 96.18 |
| F3 | 3586 | 87 | 5190 | 275 | 5.30 | 94.70 | 5192 | 204 | 3.93 | 96.07 |
| F4 | 3608 | 85 | 5118 | 249 | 4.87 | 95.13 | 5128 | 189 | 3.69 | 96.31 |
| F5 | 3591 | 82 | 4905 | 258 | 5.26 | 94.74 | 4905 | 197 | 4.02 | 95.98 |
| F6 | 3578 | 84 | 5012 | 247 | 4.93 | 95.07 | 5013 | 221 | 4.41 | 95.59 |
| F7 | 3617 | 81 | 4891 | 257 | 5.25 | 94.75 | 4893 | 198 | 4.05 | 95.95 |
| F8 | 3574 | 78 | 4653 | 255 | 5.48 | 94.52 | 4654 | 207 | 4.45 | 95.55 |
| F9 | 3652 | 85 | 5186 | 243 | 4.69 | 95.31 | 5187 | 198 | 3.82 | 96.18 |
| F10 | 3589 | 82 | 4912 | 251 | 5.11 | 94.89 | 4913 | 203 | 4.13 | 95.87 |
| Sum | 71,828 | 91,864 | 4409 | 4.77 | 95.23 | 91,889 | 3492 | 3.78 | 96.22 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nedoma, J.; Kepak, S.; Fajkus, M.; Cubik, J.; Siska, P.; Martinek, R.; Krupa, P. Magnetic Resonance Imaging Compatible Non-Invasive Fibre-Optic Sensors Based on the Bragg Gratings and Interferometers in the Application of Monitoring Heart and Respiration Rate of the Human Body: A Comparative Study. Sensors 2018, 18, 3713. https://doi.org/10.3390/s18113713
Nedoma J, Kepak S, Fajkus M, Cubik J, Siska P, Martinek R, Krupa P. Magnetic Resonance Imaging Compatible Non-Invasive Fibre-Optic Sensors Based on the Bragg Gratings and Interferometers in the Application of Monitoring Heart and Respiration Rate of the Human Body: A Comparative Study. Sensors. 2018; 18(11):3713. https://doi.org/10.3390/s18113713
Chicago/Turabian StyleNedoma, Jan, Stanislav Kepak, Marcel Fajkus, Jakub Cubik, Petr Siska, Radek Martinek, and Petr Krupa. 2018. "Magnetic Resonance Imaging Compatible Non-Invasive Fibre-Optic Sensors Based on the Bragg Gratings and Interferometers in the Application of Monitoring Heart and Respiration Rate of the Human Body: A Comparative Study" Sensors 18, no. 11: 3713. https://doi.org/10.3390/s18113713






