Indium Nitrite (InN)-Based Ultrasensitive and Selective Ammonia Sensor Using an External Silicone Oil Filter for Medical Application
Abstract
:1. Introduction
2. Method and Setup
2.1. Sampling Process
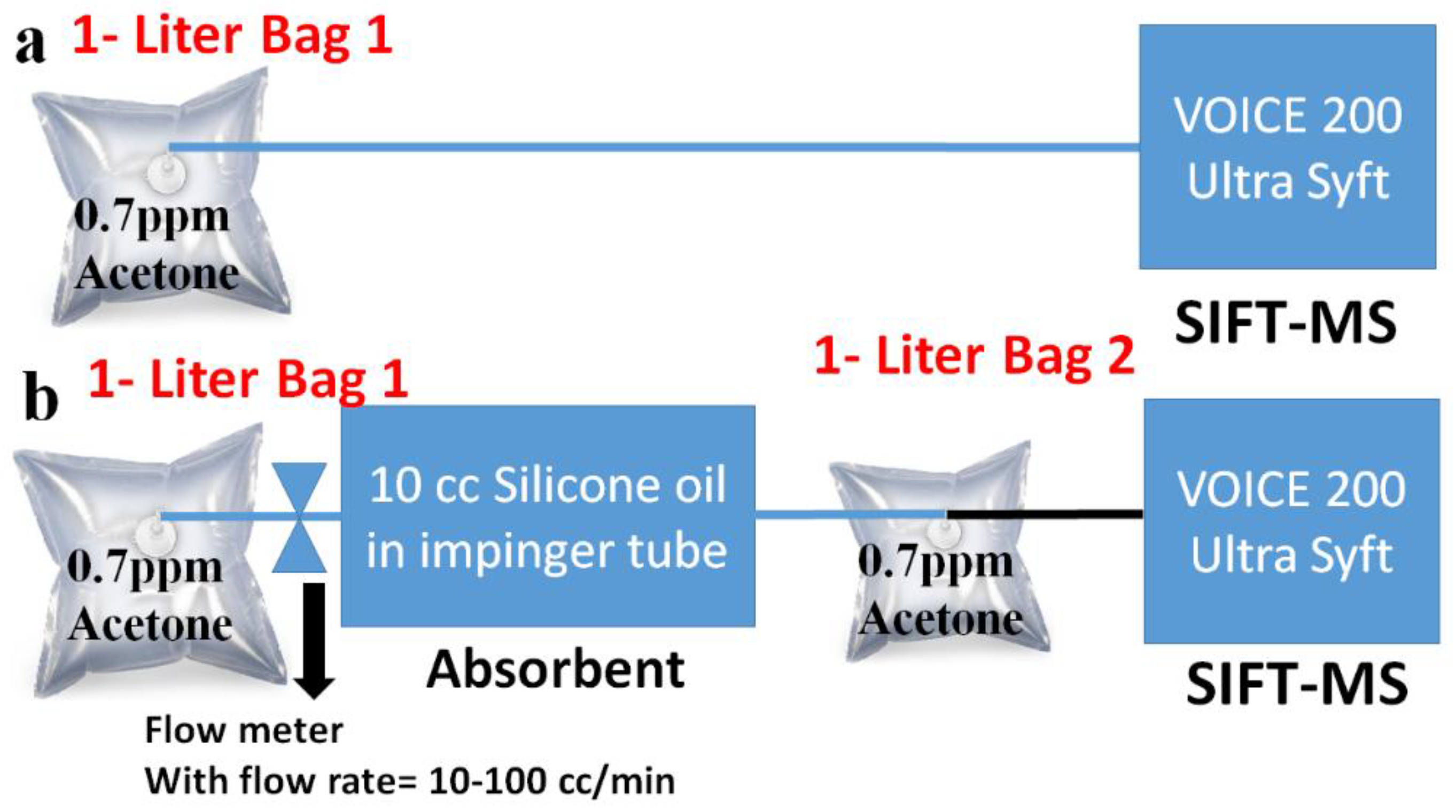
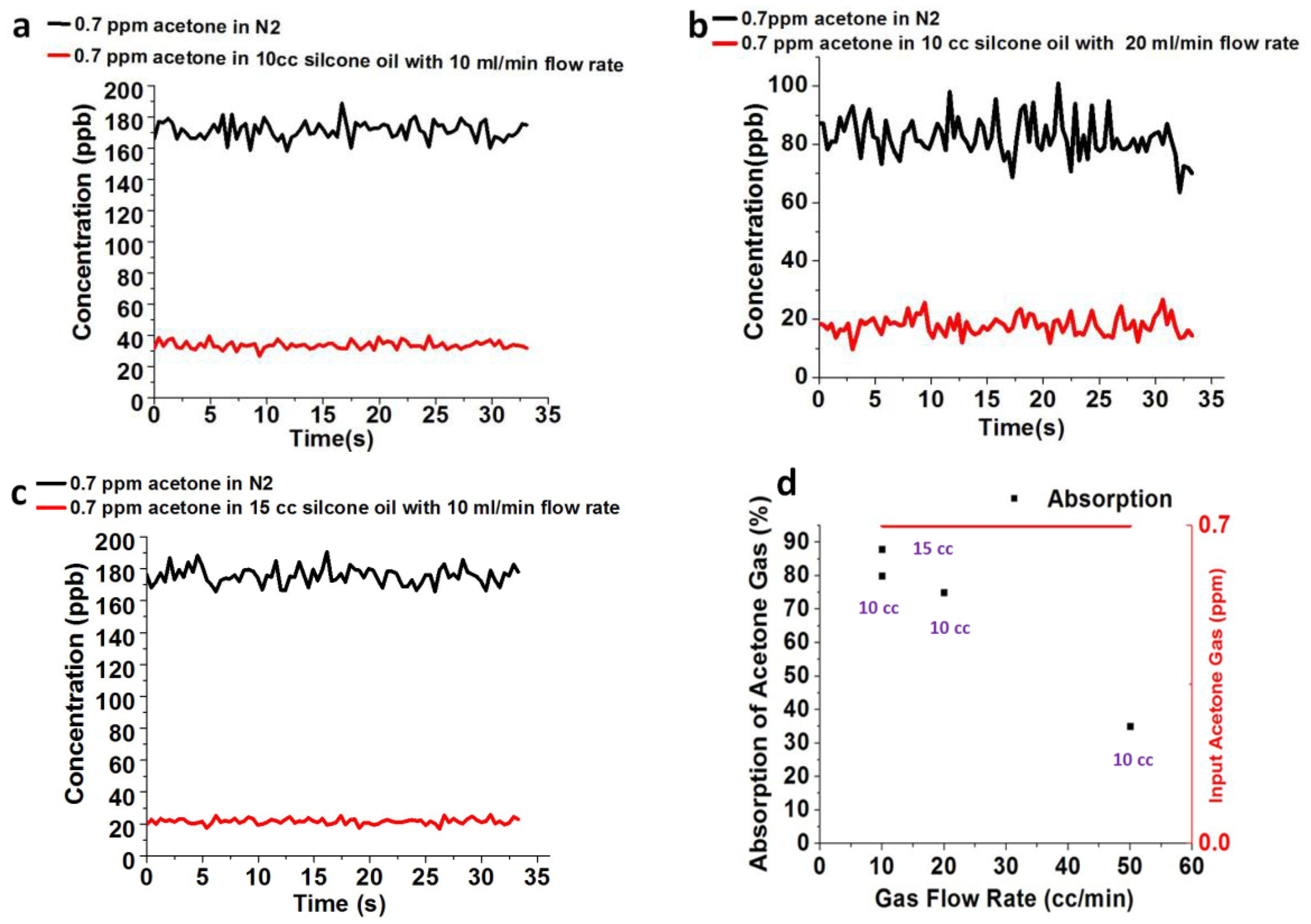
2.2. Absorption Setup of Acetone
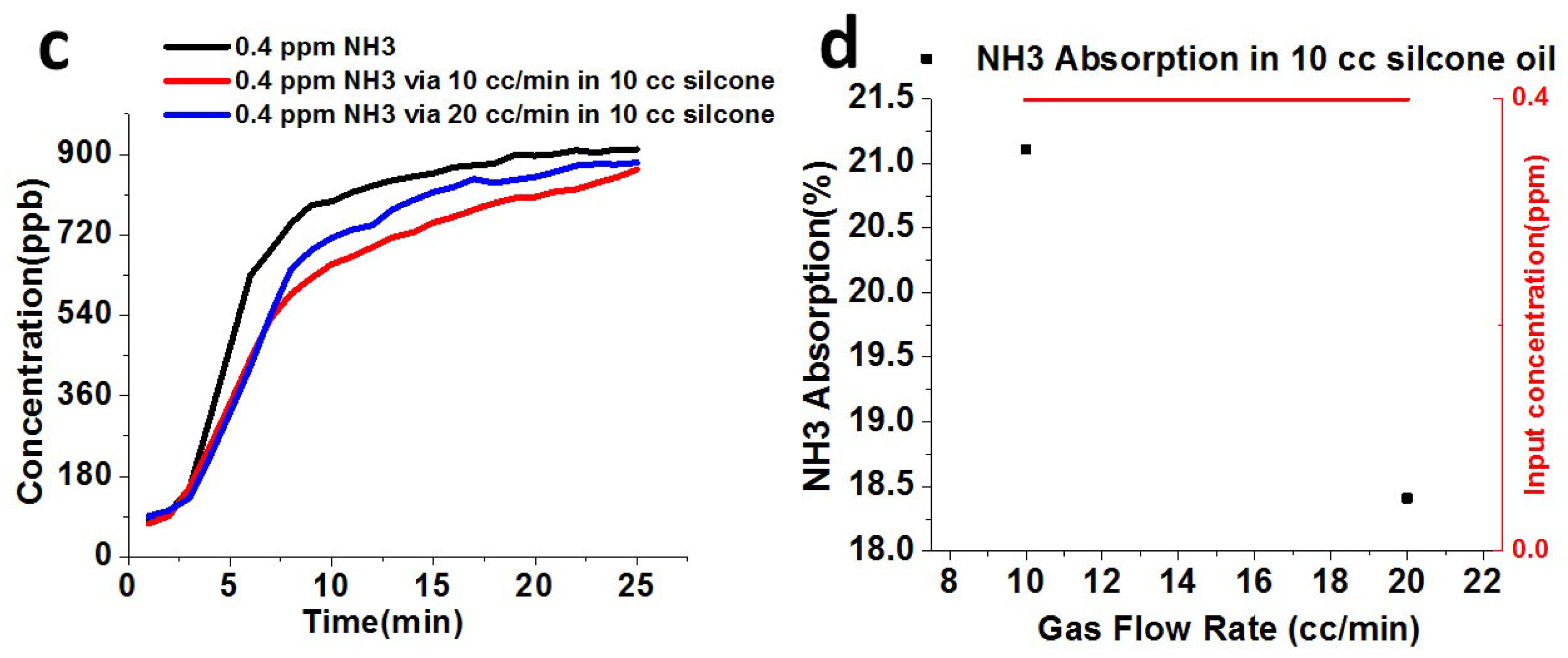
2.3. Absorption Setup of Ammonia
2.4. Sensitivity of InN Gas Sensor
3. Ultrathin InN Gas Sensor
3.1. Fabrication Process
3.2. Gas Sensor Device
Sensing Mechanism
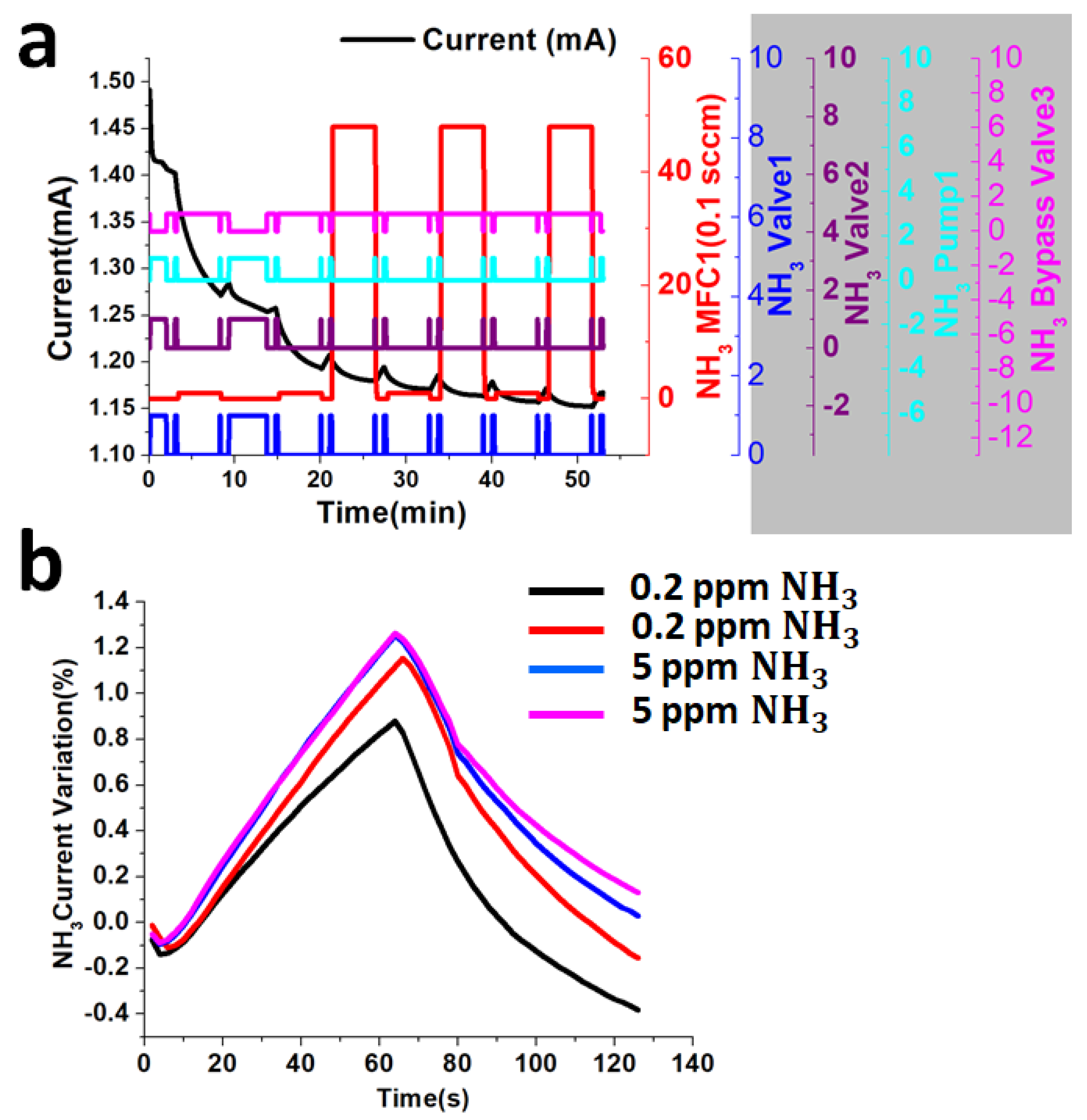
3.3. Function of Valves and Pump System
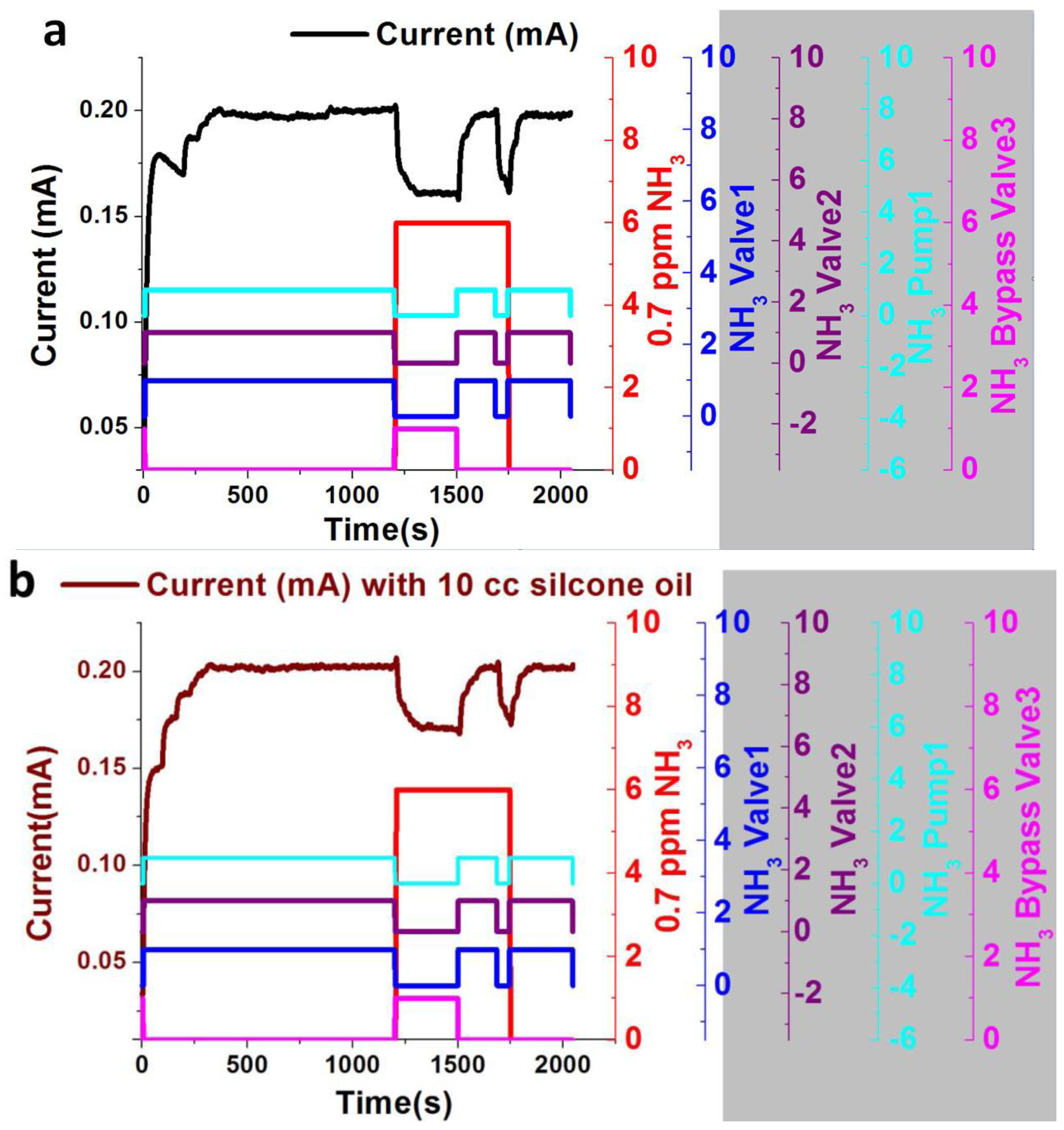
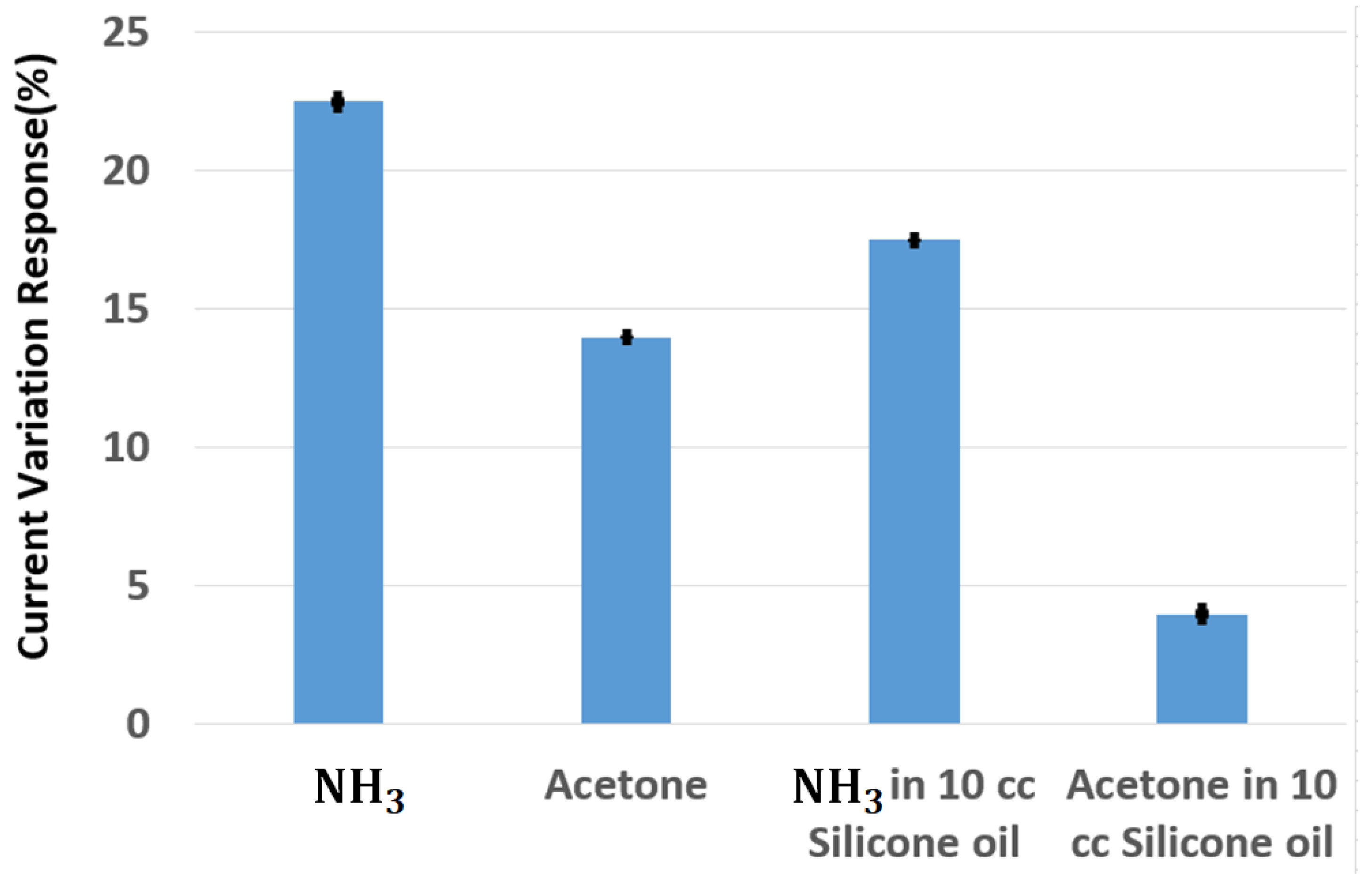
4. Results and Discussion
5. Detection Limit of Portable System
6. Conclusions
Supplementary Materials
Funding
Conflicts of Interest
References
- Das, T.; Pramanik, A.; Haldar, D. On-line Ammonia Sensor and Invisible Security Ink by Fluorescent Zwitterionic Spirocyclic Meisenheimer Complex. Sci. Rep. 2017, 7, 40465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tadi, K.K.; Pal, S.; Narayanan, T.N. Fluorographene based ultrasensitive ammonia sensor. Sci. Rep. 2016, 6, 25221. [Google Scholar] [CrossRef] [PubMed]
- Righettoni, M.; Amann, A.; Pratsinis, S.E. Breath analysis by nanostructured metal oxides as chemo-resistive gas sensors. Mater. Today 2015, 18, 163–171. [Google Scholar] [CrossRef]
- Toda, K.; Li, J.; Dasgupta, P.K. Measurement of ammonia in human breath with a liquid-film conductivity sensor. Anal. Chem. 2006, 78, 7284–7291. [Google Scholar] [CrossRef] [PubMed]
- Hibbard, T.; Crowley, K.; Kelly, F.; Ward, F.; Holian, J.; Watson, A.; Killard, A.J. Point of care monitoring of hemodialysis patients with a breath ammonia measurement device based on printed polyaniline nanoparticle sensors. Anal. Chem. 2013, 85, 12158–12165. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.-Z.; Lin, Y.-L.; Lin, H.-C.; Zan, H.-W.; Chang, K.-T.; Meng, H.-F.; Liao, J.-W.; Tsai, M.-J.; Cheng, H. Highly sensitive ammonia sensor with organic vertical nanojunctions for noninvasive detection of hepatic injury. Anal. Chem. 2013, 85, 3110–3117. [Google Scholar] [CrossRef] [PubMed]
- De Lacy Costello, B.; Amann, A.; Al-Kateb, H.; Flynn, C.; Filipiak, W.; Khalid, T.; Osborne, D.; Ratcliffe, N.M. A review of the volatiles from the healthy human body. J. Breath Res. 2014, 8, 014001. [Google Scholar] [CrossRef] [PubMed]
- Capuano, R.; Santonico, M.; Pennazza, G.; Ghezzi, S.; Martinelli, E.; Roscioni, C.; Lucantoni, G.; Galluccio, G.; Paolesse, R.; Di Natale, C. The lung cancer breath signature: A comparative analysis of exhaled breath and air sampled from inside the lungs. Sci. Rep. 2014, 5, 16491. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.; Španěl, P.; Smith, D. A longitudinal study of ammonia, acetone and propanol in the exhaled breath of 30 subjects using selected ion flow tube mass spectrometry, SIFT-MS. Physiol. Meas. 2006, 27, 321. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.; Spanel, P.; Smith, D. Quantitative analysis of ammonia on the breath of patients in end-stage renal failure. Kidney Int. 1997, 52, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Kearney, D.J.; Hubbard, T.; Putnam, D. Breath ammonia measurement in Helicobacter pylori infection. Dig. Dis. Sci. 2002, 47, 2523–2530. [Google Scholar] [CrossRef] [PubMed]
- Grabowska-Polanowska, B.; Faber, J.; Skowron, M.; Miarka, P.; Pietrzycka, A.; Śliwka, I.; Amann, A. Detection of potential chronic kidney disease markers in breath using gas chromatography with mass-spectral detection coupled with thermal desorption method. J. Chromatogr. A 2013, 1301, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Shimamoto, C.; Hirata, I.; Katsu, K. Breath and blood ammonia in liver cirrhosis. Hepato-gastroenterology 2000, 47, 443–445. [Google Scholar] [PubMed]
- Zan, H.-W.; Dai, M.-Z.; Hsu, T.-Y.; Lin, H.-C.; Meng, H.-F.; Yang, Y.-S. Porous organic TFTs for the applications on real-time and sensitive gas sensors. IEEE Electron Device Lett. 2011, 32, 1143–1145. [Google Scholar] [CrossRef]
- Xing, R.; Xu, L.; Song, J.; Zhou, C.; Li, Q.; Liu, D.; Song, H.W. Preparation and gas sensing properties of In2O3/Au nanorods for detection of volatile organic compounds in exhaled breath. Sci. Rep. 2015, 5, 10717. [Google Scholar] [CrossRef] [PubMed]
- Ryabtsev, S.; Shaposhnick, A.; Lukin, A.; Domashevskaya, E. Application of semiconductor gas sensors for medical diagnostics. Sens. Actuators B Chem. 1999, 59, 26–29. [Google Scholar] [CrossRef]
- Teshima, N.; Li, J.; Toda, K.; Dasgupta, P.K. Determination of acetone in breath. Anal. Chim. Acta 2005, 535, 189–199. [Google Scholar] [CrossRef]
- Shin, J.; Choi, S.J.; Lee, I.; Youn, D.Y.; Park, C.O.; Lee, J.H.; Tuller, H.L.; Kim, I.D. Thin-wall assembled SnO2 fibers functionalized by catalytic Pt nanoparticles and their superior exhaled-breath-sensing properties for the diagnosis of diabetes. Adv. Funct. Mater. 2013, 23, 2357–2367. [Google Scholar] [CrossRef]
- Abbott, S.M.; Elder, J.B.; Španěl, P.; Smith, D. Quantification of acetonitrile in exhaled breath and urinary headspace using selected ion flow tube mass spectrometry. Int. J. Mass Spectrom. 2003, 228, 655–665. [Google Scholar] [CrossRef]
- Van den Velde, S.; Nevens, F.; van Steenberghe, D.; Quirynen, M. GC–MS analysis of breath odor compounds in liver patients. J. Chromatogr. B 2008, 875, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Tangerman, A.; Meuwese-Arends, M.T.; van Tongeren, J.H. A new sensitive assay for measuring volatile sulphur compounds in human breath by Tenax trapping and gas chromatography and its application in liver cirrhosis. Clin. Chim. Acta 1983, 130, 103–110. [Google Scholar] [CrossRef]
- Španěl, P.; Davies, S.; Smith, D. Quantification of breath isoprene using the selected ion flow tube mass spectrometric analytical method. Rapid Commun. Mass Spectrom. 1999, 13, 1733–1738. [Google Scholar] [CrossRef]
- Diskin, A.M.; Španěl, P.; Smith, D. Time variation of ammonia, acetone, isoprene and ethanol in breath: A quantitative SIFT-MS study over 30 days. Physiol. Meas. 2003, 24, 107. [Google Scholar] [CrossRef] [PubMed]
- Nelson, N.; Lagesson, V.; Nosratabadi, A.R.; Ludvigsson, J.; Tagesson, C. Exhaled isoprene and acetone in newborn infants and in children with diabetes mellitus. Pediatr. Res. 1998, 44, 363. [Google Scholar] [CrossRef] [PubMed]
- Konvalina, G.; Haick, H. Sensors for breath testing: From nanomaterials to comprehensive disease detection. Acc. Chem. Res. 2013, 47, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Tisch, U.; Haick, H. Nanomaterials for cross-reactive sensor arrays. MRS Bull. 2010, 35, 797–803. [Google Scholar] [CrossRef]
- Broza, Y.Y.; Haick, H. Nanomaterial-based sensors for detection of disease by volatile organic compounds. Nanomedicine 2013, 8, 785–806. [Google Scholar] [CrossRef] [PubMed]
- Lupan, O.; Schütt, F.; Postica, V.; Smazna, D.; Mishra, Y.K.; Adelung, R. Sensing performances of pure and hybridized carbon nanotubes-ZnO nanowire networks: A detailed study. Sci. Rep. 2017, 7, 14715. [Google Scholar] [CrossRef] [PubMed]
- Lupan, O.; Wolff, N.; Postica, V.; Braniste, T.; Paulowicz, I.; Hrkac, V.; Mishra, Y.K.; Tiginyanu, I.; Kienle, L.; Adelung, R. Properties of a single SnO2: Zn2SnO4–Functionalized nanowire based nanosensor. Ceram. Int. 2017, 44, 4859–4867. [Google Scholar] [CrossRef]
- Lupan, O.; Postica, V.; Marx, J.; Mecklenburg, M.; Mishra, Y.K.; Schulte, K.; Fiedler, B.; Adelung, R. Individual hollow and mesoporous aero-graphitic microtube based devices for gas sensing applications. Appl. Phys. Lett. 2017, 110, 263109. [Google Scholar] [CrossRef]
- Mishra, Y.K.; Adelung, R. ZnO tetrapod materials for functional applications. Mater. Today 2017, 21, 631–651. [Google Scholar] [CrossRef]
- Muhulet, A.; Miculescu, F.; Voicu, S.I.; Schütt, F.; Thakur, V.K.; Mishra, Y.K. Fundamentals and scopes of doped carbon nanotubes towards energy and biosensing applications. Mater. Today Energy 2018, 9, 154–186. [Google Scholar] [CrossRef]
- Malik, R.; Tomer, V.K.; Dankwort, T.; Mishra, Y.K.; Kienle, L. Cubic mesoporous Pd–WO3 loaded graphitic carbon nitride (g-CN) nanohybrids: Highly sensitive and temperature dependent VOC sensors. J. Mater. Chem. A 2018, 6, 10718–10730. [Google Scholar] [CrossRef]
- Smazna, D.; Rodrigues, J.; Shree, S.; Postica, V.; Neubüser, G.; Martins, A.; Sedrine, N.B.; Jena, N.K.; Siebert, L.; Schütt, F. Buckminsterfullerene hybridized zinc oxide tetrapods: Defects and charge transfer induced optical and electrical response. Nanoscale 2018, 10, 10050–10062. [Google Scholar] [CrossRef] [PubMed]
- Gouma, P.; Kalyanasundaram, K.; Yun, X.; Stanacevic, M.; Wang, L. Nanosensor and breath analyzer for ammonia detection in exhaled human breath. IEEE Sens. J. 2010, 10, 49–53. [Google Scholar] [CrossRef]
- Samotaev, N.; Podlepetsky, B.; Vasiliev, A.; Pisliakov, A.; Sokolov, A. Metal-oxide gas sensor high-selective to ammonia. Autom. Remote Control 2013, 74, 308–312. [Google Scholar] [CrossRef]
- Chatterjee, B.; Bandyopadhyay, A. Development of zinc oxide sensors for detecting ammonia gas in the ambient air: A critical short review. Environ. Qual. Manag. 2016, 26, 89–105. [Google Scholar] [CrossRef]
- Aslam, M.; Chaudhary, V.; Mulla, I.; Sainkar, S.; Mandale, A.; Belhekar, A.; Vijayamohanan, K. A highly selective ammonia gas sensor using surface-ruthenated zinc oxide. Sens. Actuators A Phys. 1999, 75, 162–167. [Google Scholar] [CrossRef]
- Chen, H.-I.; Hsiao, C.-Y.; Chen, W.-C.; Chang, C.-H.; Chou, T.-C.; Liu, I.-P.; Lin, K.-W.; Liu, W.-C. Characteristics of a Pt/NiO thin film-based ammonia gas sensor. Sens. Actuators B Chem. 2018, 256, 962–967. [Google Scholar] [CrossRef]
- Navaneethan, M.; Patil, V.; Ponnusamy, S.; Muthamizhchelvan, C.; Kawasaki, S.; Patil, P.; Hayakawa, Y. Sensitivity enhancement of ammonia gas sensor based on Ag/ZnO flower and nanoellipsoids at low temperature. Sens. Actuators B Chem. 2018, 255, 672–683. [Google Scholar]
- Liu, X.; Cheng, S.; Liu, H.; Hu, S.; Zhang, D.; Ning, H. A survey on gas sensing technology. Sensors 2012, 12, 9635–9665. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, A.G.; Hashimoto, A.; Yamamoto, A. Indium nitride (InN): A review on growth, characterization, and properties. J. Appl. Phys. 2003, 94, 2779–2808. [Google Scholar] [CrossRef]
- Lu, H.; Schaff, W.J.; Eastman, L.F.; Stutz, C. Surface charge accumulation of InN films grown by molecular-beam epitaxy. Appl. Phys. Lett. 2003, 82, 1736–1738. [Google Scholar] [CrossRef]
- Rickert, K.A.; Ellis, A.B.; Himpsel, F.J.; Lu, H.; Schaff, W.; Redwing, J.M.; Dwikusuma, F.; Kuech, T.F. X-ray photoemission spectroscopic investigation of surface treatments, metal deposition, and electron accumulation on InN. Appl. Phys. Lett. 2003, 82, 3254–3256. [Google Scholar] [CrossRef]
- Veal, T.; Mahboob, I.; Piper, L.; McConville, C.; Lu, H.; Schaff, W. Indium nitride: Evidence of electron accumulation. J. Vac. Sci. Technol. B 2004, 22, 2175–2178. [Google Scholar] [CrossRef]
- Mahboob, I.; Veal, T.; Piper, L.; McConville, C.; Lu, H.; Schaff, W.; Furthmüller, J.; Bechstedt, F. Origin of electron accumulation at wurtzite InN surfaces. Phys. Rev. B 2004, 69, 201307. [Google Scholar] [CrossRef]
- Mahboob, I.; Veal, T.; McConville, C.; Lu, H.; Schaff, W. Intrinsic electron accumulation at clean InN surfaces. Phys. Rev. Lett. 2004, 92, 036804. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.P.; Reedy, B.J. Temperature modulation in semiconductor gas sensing. Sens. Actuators B Chem. 1999, 60, 35–42. [Google Scholar] [CrossRef]
- Chakraborty, S.; Sen, A.; Maiti, H.S. Selective detection of methane and butane by temperature modulation in iron doped tin oxide sensors. Sens. Actuators B Chem. 2006, 115, 610–613. [Google Scholar] [CrossRef]
- Nakata, S.; Okunishi, H.; Nakashima, Y. Distinction of gases with a semiconductor sensor depending on the scanning profile of a cyclic temperature. Analyst 2006, 131, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Cavicchi, R.; Suehle, J.; Kreider, K.; Gaitan, M.; Chaparala, P. Fast temperature programmed sensing for micro-hotplate gas sensors. IEEE Electron Device Lett. 1995, 16, 286–288. [Google Scholar] [CrossRef]
- Yu, J.H.; Choi, G.M. Selective CO gas detection of CuO-and ZnO-doped SnO2 gas sensor. Sens. Actuators B Chem. 2001, 75, 56–61. [Google Scholar] [CrossRef]
- Heilig, A.; Barsan, N.; Weimar, U.; Schweizer-Berberich, M.; Gardner, J.; Göpel, W. Gas identification by modulating temperatures of SnO2-based thick film sensors. Sens. Actuators B Chem. 1997, 43, 45–51. [Google Scholar] [CrossRef]
- Kunt, T.A.; McAvoy, T.J.; Cavicchi, R.E.; Semancik, S. Optimization of temperature programmed sensing for gas identification using micro-hotplate sensors. Sens. Actuators B Chem. 1998, 53, 24–43. [Google Scholar] [CrossRef]
- Di Natale, C.; Macagnano, A.; Martinelli, E.; Paolesse, R.; D’Arcangelo, G.; Roscioni, C.; Finazzi-Agrò, A.; D’Amico, A. Lung cancer identification by the analysis of breath by means of an array of non-selective gas sensors. Biosens. Bioelectron. 2003, 18, 1209–1218. [Google Scholar] [CrossRef]
- Star, A.; Joshi, V.; Skarupo, S.; Thomas, D.; Gabriel, J.-C.P. Gas sensor array based on metal-decorated carbon nanotubes. J. Phys. Chem. B 2006, 110, 21014–21020. [Google Scholar] [CrossRef] [PubMed]
- Sysoev, V.V.; Goschnick, J.; Schneider, T.; Strelcov, E.; Kolmakov, A. A gradient microarray electronic nose based on percolating SnO2 nanowire sensing elements. Nano Lett. 2007, 7, 3182–3188. [Google Scholar] [CrossRef] [PubMed]
- Röck, F.; Barsan, N.; Weimar, U. Electronic nose: Current status and future trends. Chem. Rev. 2008, 108, 705–725. [Google Scholar] [CrossRef] [PubMed]
- Kao, K.-W.; Hsu, M.-C.; Chang, Y.-H.; Gwo, S.; Yeh, J.A. A sub-ppm acetone gas sensor for diabetes detection using 10 nm thick ultrathin InN FETs. Sensors 2012, 12, 7157–7168. [Google Scholar] [CrossRef] [PubMed]
- Kao, K.-W.; Su, Y.-W.; Lu, Y.-S.; Gwo, S.; Yeh, J.A. Calcium ion detection using miniaturized InN-based ion sensitive field effect transistors. Int. J. Autom. Smart Technol. 2012, 2, 49–54. [Google Scholar] [CrossRef]
- Kao, K.-W.A.; Cheng, C.-J.; Gwo, S.; Yeh, J.A. A semiconductor gas system of healthcare for liver disease detection using ultrathin InN-based sensor. ECS Trans. 2015, 66, 151–157. [Google Scholar] [CrossRef]
- Poddar, T.K. Removal of VOCs from Air by Absorption and Stripping in Hollow Fiber Devices. Ph.D. Thesis, New Jersey Institute of Technology, Department of Chemical Engineering, Chemistry and Environmental Sciemce, Newark, NJ, USA, 1995. [Google Scholar]
- Wedlake, G.D.; Robinson, D.B. Solubility of Carbon dioxide in silicone Oil. J. Chem. Eng. Data 1979, 24, 305–306. [Google Scholar] [CrossRef]
- Bikov, A.; Paschalaki, K.; Logan-Sinclair, R.; Horváth, I.; Kharitonov, S.A.; Barnes, P.J.; Usmani, O.S.; Paredi, P. Standardised exhaled breath collection for the measurement of exhaled volatile organic compounds by proton transfer reaction mass spectrometry. BMC Pulm. Med. 2013, 13, 43. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Hoffman, L.A.; Sethi, J.M.; Zullo, T.G.; Gibson, K.F. Multiple flow rates measurement of exhaled nitric oxide in patients with sarcoidosis: A pilot feasibility study. Sarcoidosis Vasc. Diffus. Lung Dis. 2009, 26, 98. [Google Scholar]
- Elperin, T.; Fominykh, A. Model of gas absorption in gas-liquid plug flow with first-order and zero-order chemical reaction. Heat Mass Transf. 2003, 39, 195–199. [Google Scholar] [CrossRef]
- Elperin, T.; Fominykh, A. Mass transfer during gas absorption in a vertical gas-liquid slug flow with small bubbles in liquid plugs. Heat Mass Transf. 1998, 33, 489–494. [Google Scholar] [CrossRef]
- Rangel-Kuoppa, V.-T.; Suihkonen, S.; Sopanen, M.; Lipsanen, H. Metal contacts on InN: Proposal for Schottky contact. Jpn. J. Appl. Phys. 2006, 45, 36. [Google Scholar] [CrossRef]
- Sai, P. Ohmic contacts to InN-based materials. Tekhnol. Konstr. Elektron. Appar. 2016, 3–14. [Google Scholar] [CrossRef]
- Golunski, S. Why use platinum in catalytic converters. Platin. Met. Rev. 2007, 51, 162. [Google Scholar] [CrossRef]
- Sealy, C. The problem with platinum. Mater. Today 2008, 11, 65–68. [Google Scholar] [CrossRef]
- Cheng, C.-C.; Tsai, Y.-Y.; Lin, K.-W.; Chen, H.-I.; Hsu, W.-H.; Hong, C.-W.; Lin, H.-L.; Liu, W.-C. Study of hydrogen-sensing characteristics of a Pt-oxide-AlGaAs metal-oxide-semiconductor high electron mobility transistor. J. Vac. Sci. Technol. B 2005, 23, 1943–1947. [Google Scholar] [CrossRef]
- Barsan, N.; Weimar, U. Conduction model of metal oxide gas sensors. J. Electroceram. 2001, 7, 143–167. [Google Scholar] [CrossRef]
- Chen, T.-Y.; Chen, H.-I.; Liu, Y.-J.; Huang, C.-C.; Hsu, C.-S.; Chang, C.-F.; Liu, W.-C. Ammonia sensing properties of a Pt/AlGaN/GaN Schottky diode. IEEE Trans. Electron Devices 2011, 58, 1541–1547. [Google Scholar] [CrossRef]
- Lundström, K.; Shivaraman, M.; Svensson, C. A hydrogen-sensitive Pd-gate MOS transistor. J. Appl. Phys. 1975, 46, 3876–3881. [Google Scholar] [CrossRef]
- Lundström, I.; Shivaraman, S.; Svensson, C.; Lundkvist, L. A hydrogen—sensitive MOS field—effect transistor. Appl. Phys. Lett. 1975, 26, 55–57. [Google Scholar] [CrossRef]
- Lundström, I.; Shivaraman, M.; Svensson, C. Chemical reactions on palladium surfaces studied with Pd-MOS structures. Surf. Sci. 1977, 64, 497–519. [Google Scholar] [CrossRef]








| Symbols | Name |
|---|---|
| Mobility | |
| Electron density in ambient gas | |
| Electronics charge | |
| Electric field | |
| Cross sectional area | |
| Electron density in presence of analyte gas |
| Pt Catalyst | Advantage [70] | Disadvantage [71] |
|---|---|---|
| High melting point | Cost is very high | |
| Has ability to interact with poisons and Sulfur compound | Limited supply | |
| Efficiently recycled | Geological singularity |
| Parameter | Before Absorption | After Absorption | Silicone Oil | Flow Rate |
|---|---|---|---|---|
| Detection limit NH3 | 0.2 ppm | 0.242 ppm | 10 cc | 10 cc/min |
| Detection limit of Acetone | 0.2 ppm | 0.36 ppm | 10 cc | 10 cc/min |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rai, S.K.; Kao, K.-W.; Gwo, S.; Agarwal, A.; Lin, W.D.; Yeh, J.A. Indium Nitrite (InN)-Based Ultrasensitive and Selective Ammonia Sensor Using an External Silicone Oil Filter for Medical Application. Sensors 2018, 18, 3887. https://doi.org/10.3390/s18113887
Rai SK, Kao K-W, Gwo S, Agarwal A, Lin WD, Yeh JA. Indium Nitrite (InN)-Based Ultrasensitive and Selective Ammonia Sensor Using an External Silicone Oil Filter for Medical Application. Sensors. 2018; 18(11):3887. https://doi.org/10.3390/s18113887
Chicago/Turabian StyleRai, Sujeet Kumar, Kun-Wei Kao, Shanjgr Gwo, Ashish Agarwal, Wei Da Lin, and J. Andrew Yeh. 2018. "Indium Nitrite (InN)-Based Ultrasensitive and Selective Ammonia Sensor Using an External Silicone Oil Filter for Medical Application" Sensors 18, no. 11: 3887. https://doi.org/10.3390/s18113887





