Design, Modeling, and Evaluation of the Eddy Current Sensor Deeply Implanted in the Human Body
Abstract
:1. Introduction
1.1. Osseointegration, Implant Stability, and Micromotion
1.2. Modalities to Measure the Micromotion
2. Methods
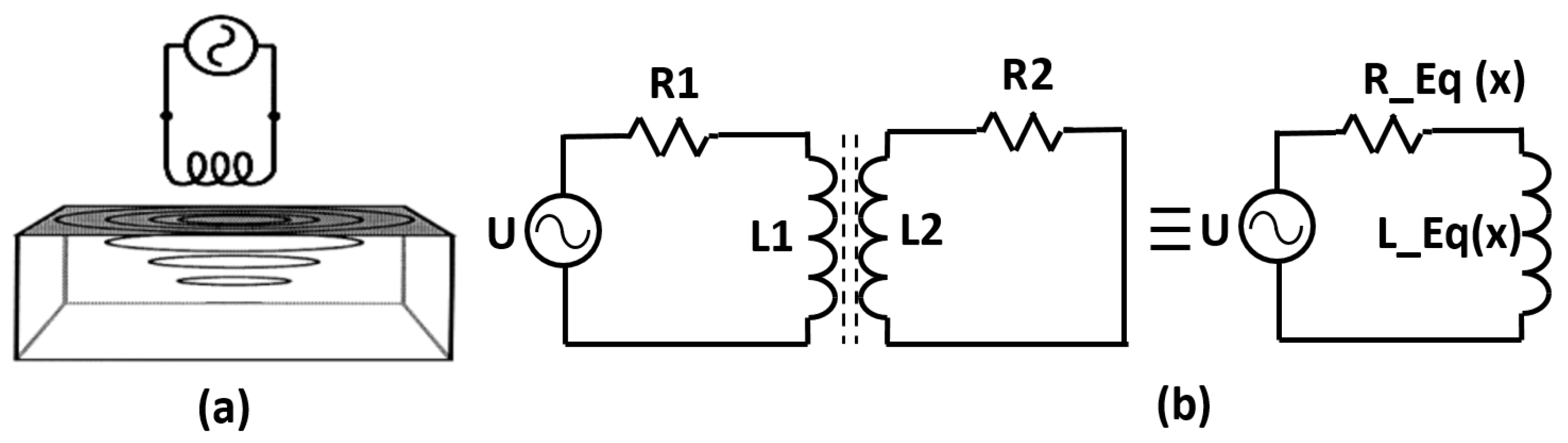
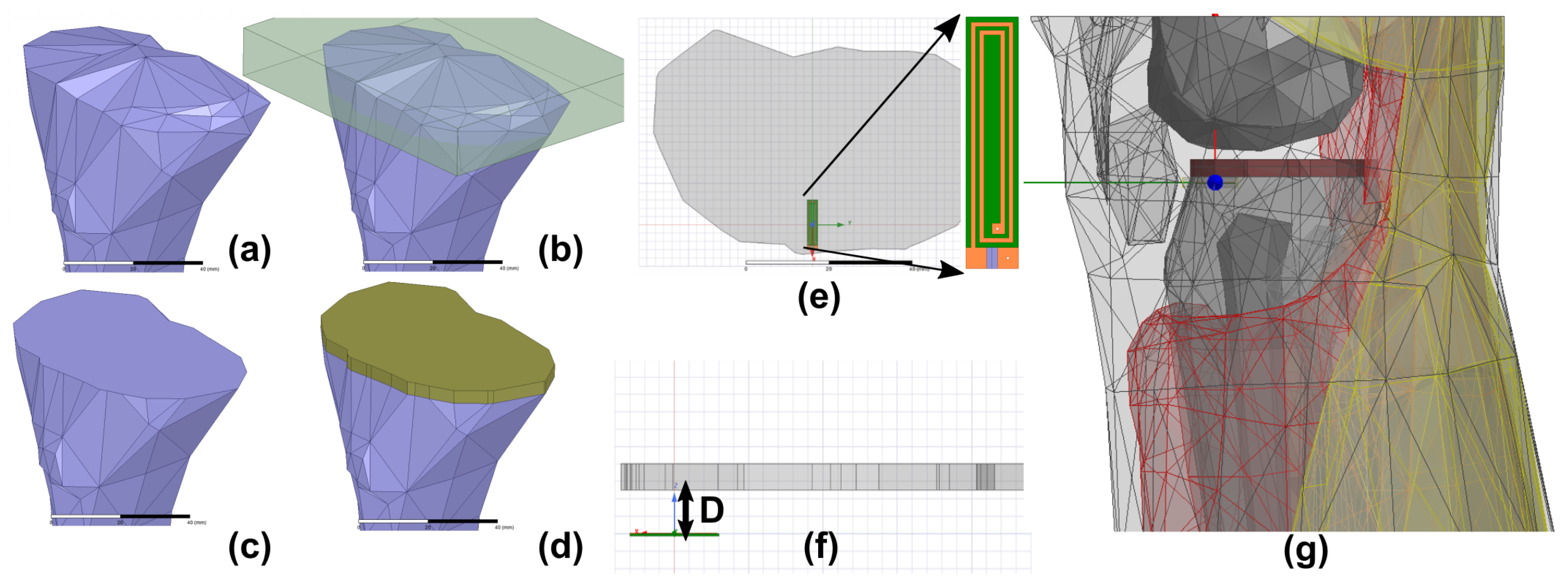
2.1. Numerical Modeling of Sensors
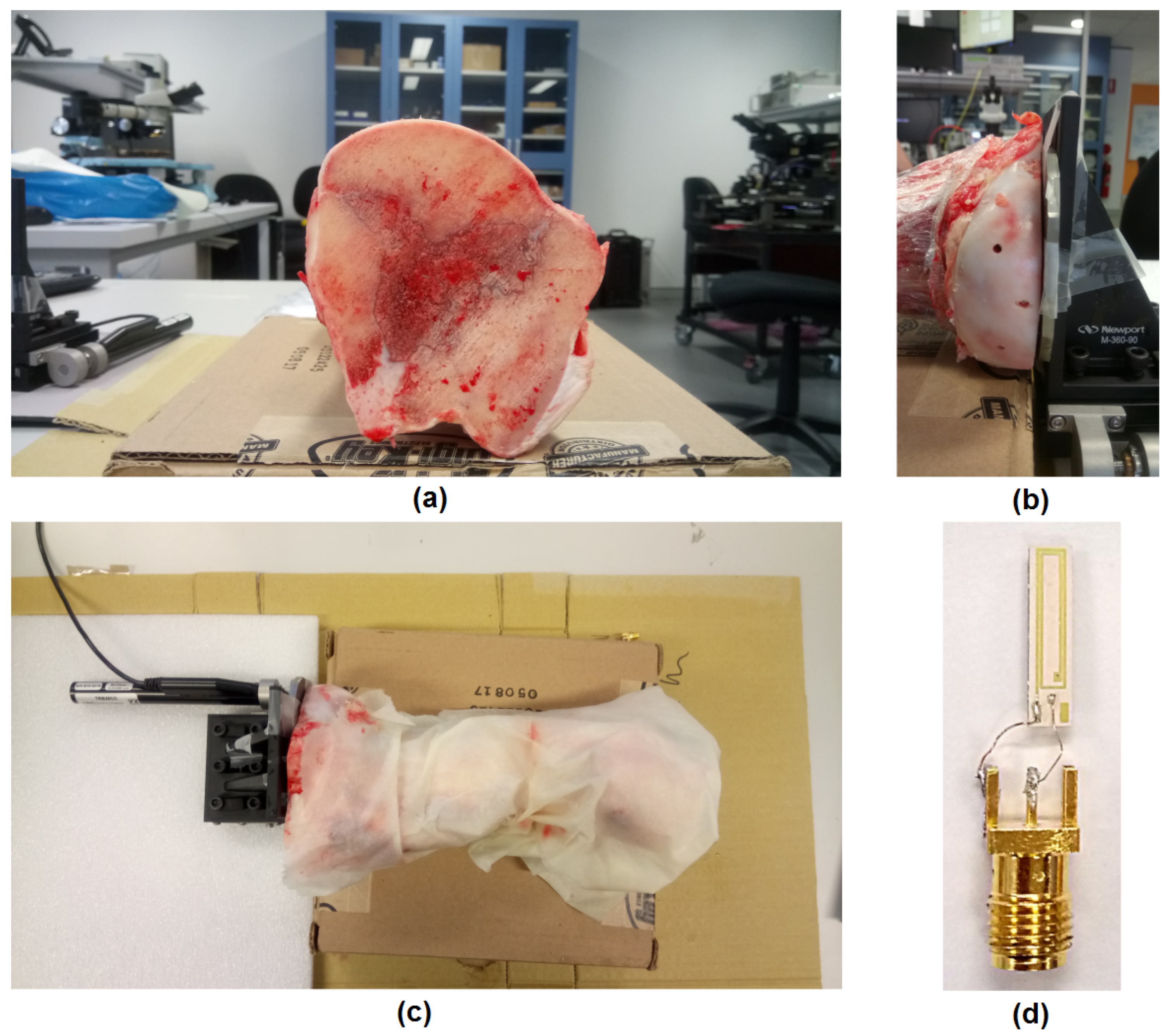
2.2. Experimental Setup
3. Results and Analysis
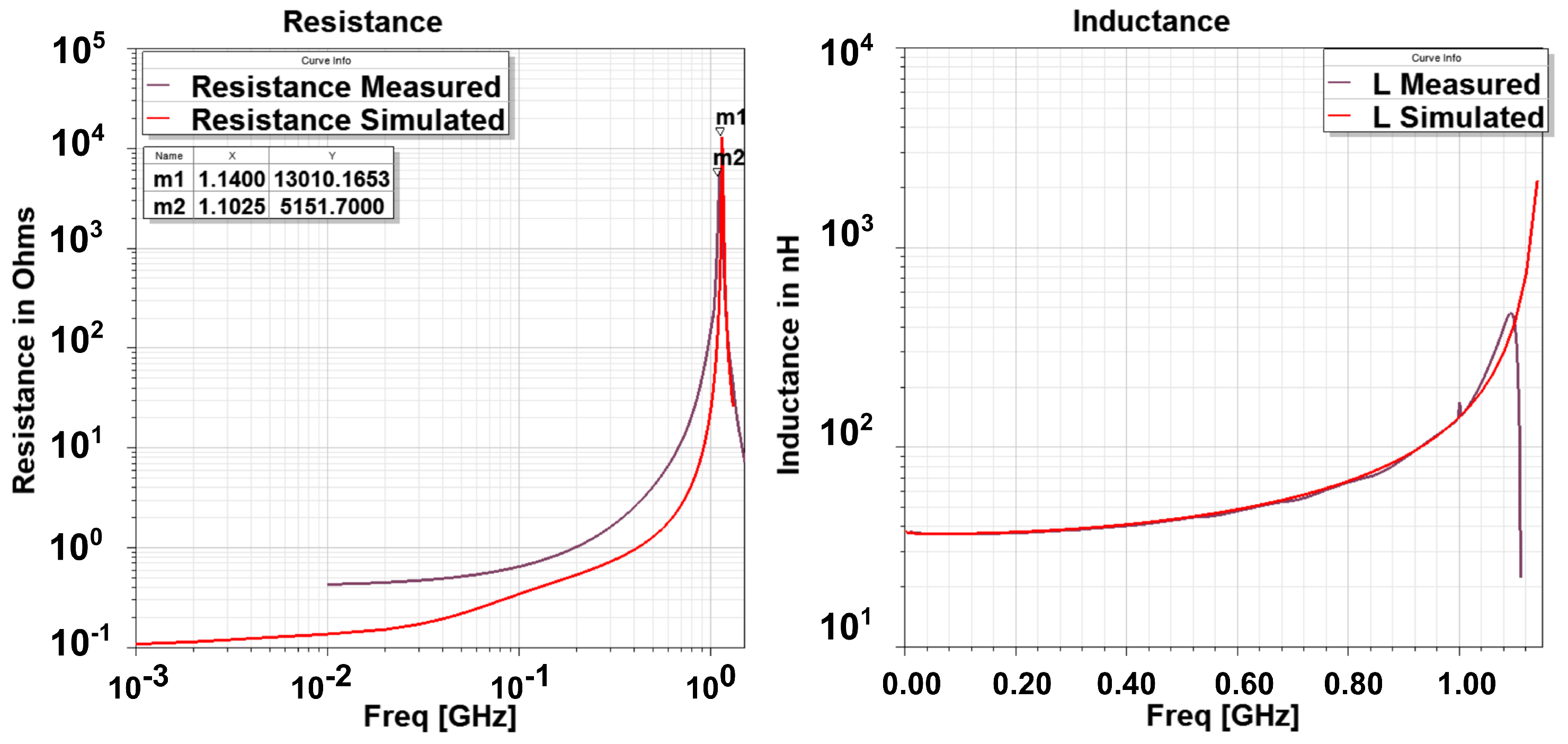
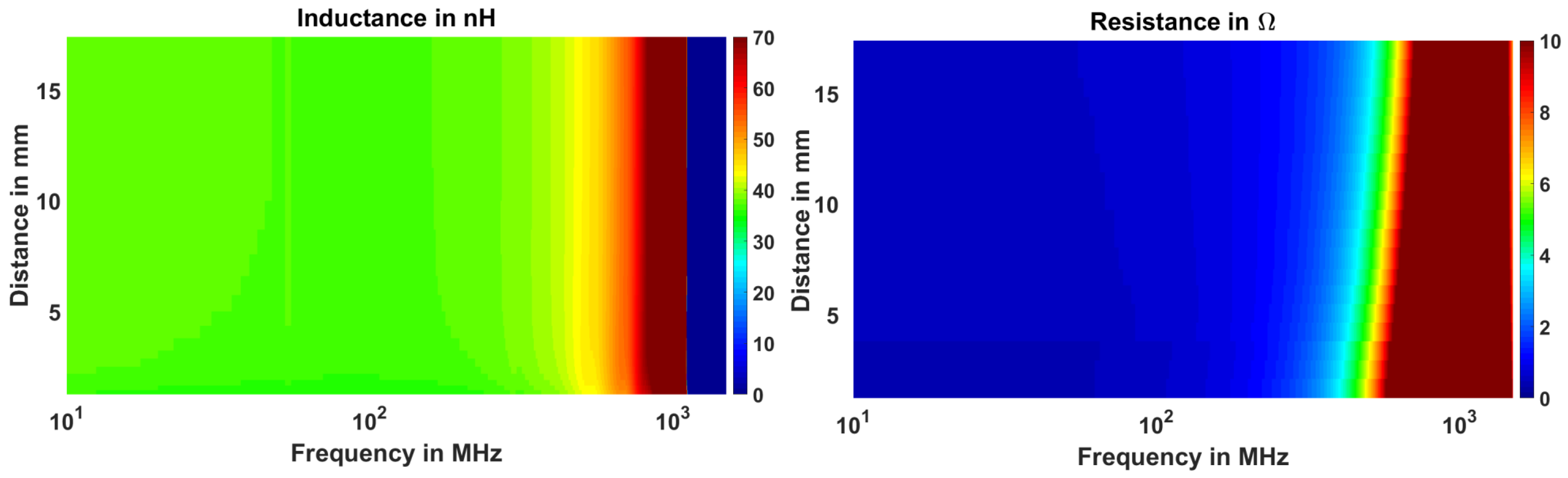
3.1. Numerical and Experimental Results in Free Space
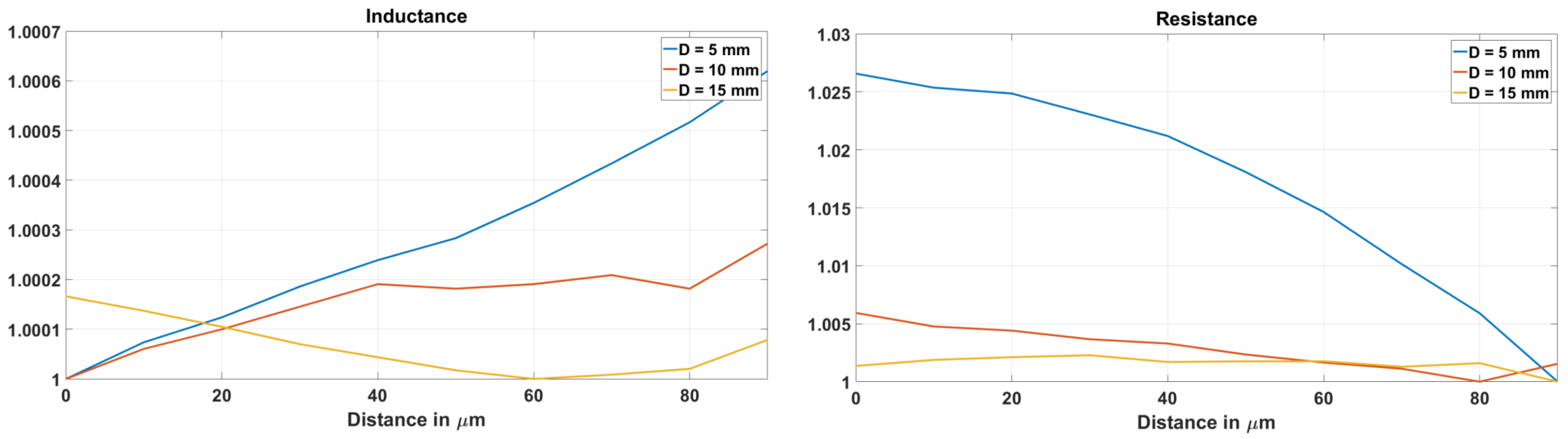
3.2. Experiments in a Cow Bone
3.3. Evaluation of the Sensitivity of EC Sensor
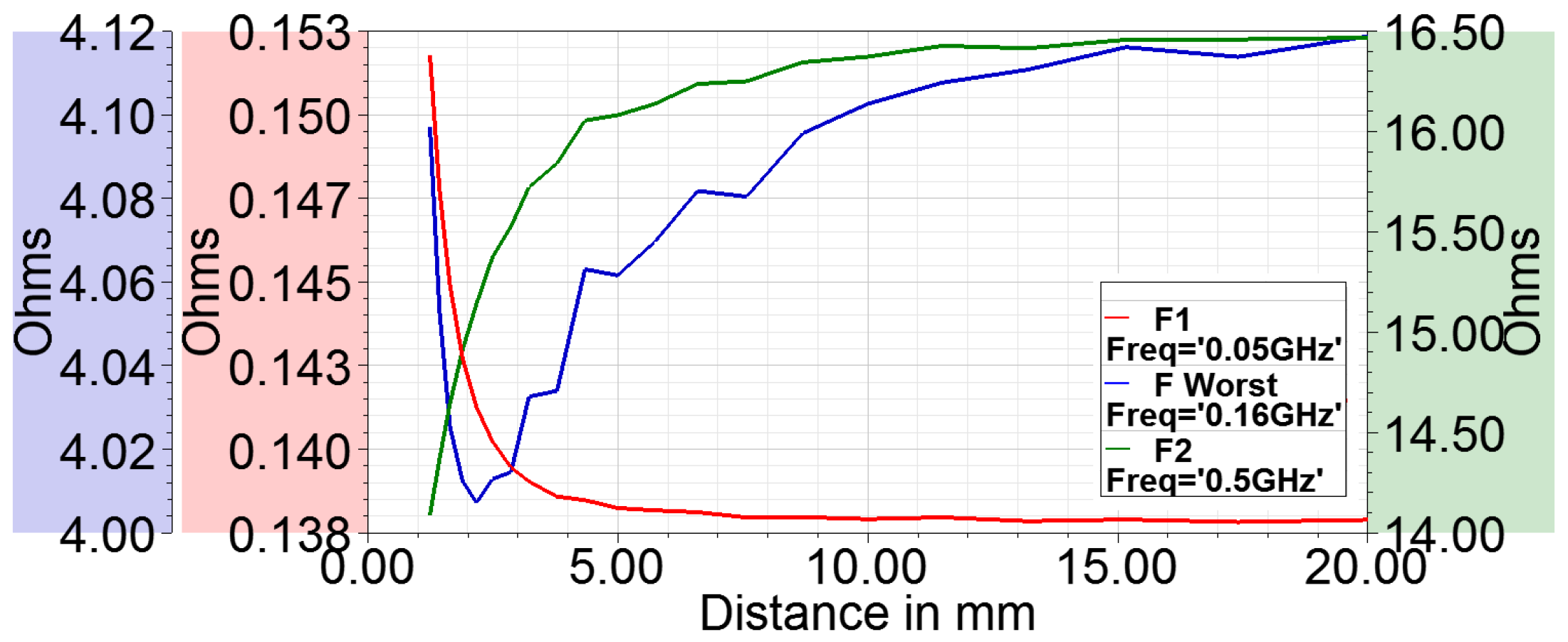
- The curve fit at maximum frequency points is exceptionally good as seen from adjusted graph where its value is near to 1.
- At , the adjusted drops to a low value for the three parameters. This indicates a failed curve fit. Correspondingly, the curve fit coefficients have discontinuity or abrupt change at and so are not reliable indicators of the actual behavior. This behavior is expected as the resistance becomes infinite and the reactance abruptly changes to negative value; therefore, there are no current flows through the loop and thus the curve fit fails.
- The adjusted drops at a certain frequency before SRF for resistance (and so the Q factor). It is around 160 MHz. This also indicates that curve fit does not reproduce the data points well. In addition, it can be noted that at this frequency (), co-efficient a becomes negative for resistance. Co-efficient c always remains positive which merely indicates that the resistance is positive.
3.4. Definition of EC Sensor Parameters
4. Future Directions
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| TKA | Total Knee Arthroplasty |
| TKR | Total Knee Replacement |
| EC | Eddy Current |
References
- Australian Orthopaedic Association Hip and Knee Arthroplasty Annual Report; Adelaide, Australia, 2015; Available online: https://aoanjrr.sahmri.com/annual-reports-2015 (accessed on 7 December 2016).
- Australian Orthopaedic Association Shoulder Arthroplasty Annual Report; Adelaide, Australia, 2015; Available online: https://aoanjrr.sahmri.com/annual-reports-2015 (accessed on 7 December 2016).
- The New Zealand Joint Registery Annual Report; Wellington, New Zealand, 2015; Available online: https://nzoa.org.nz/system/files/NZJR2014Report.pdf (accessed on 11 December 2016).
- 12th Annual National Joint Registry Report for England, Wales, Northern Ireland and the Isle of Man; London, UK, 2015; Available online: https://www.hqip.org.uk/resource/national-joint-registry-12th-annual-report-2015/#.W-P_rfYRVKI (accessed on 11 December 2016).
- 1st Annual Report, Portugese Arthroplasty Register; Parque das Nacoes 1990-024 Lisboa Portugal; 2015; Available online: http://www.rpa.spot.pt/Quick-Links/Home/RPA_first_anual_report.aspx (accessed on 11 December 2016).
- Online LROI Annual Report Dutch Arthroplasty Register. Available online: http://www.lroi-rapportage.nl (accessed on 11 December 2016).
- American Joint Replacement Registry Annual Report; Rosemont, IL, USA, 2014; Available online: http://www.ajrr.net/images/annual_reports/AJRR_2014_Annual_Report_final_11-11-15.pdf (accessed on 7 December 2016).
- SIRIS Report 2012–2015, Annual Report of the Swiss National Joint Registry, Hip and Knee; National Association for Quality Development in Hospitals and Clinics: Bern, Switzerland, 2014; Available online: http://www.swissorthopaedics.ch/images/content/SIRIS/170516_SIRISAnnualReport2015_Finalcopie.pdf (accessed on 11 December 2016).
- Orthopride Belgian Hip and Knee Arthroplasty Registry Annual Report 2014; Belgium, December 2015. Available online: https://www.ehealth.fgov.be/file/view/5fca367bad5fd36bf017d1363087849a (accessed on 11 December 2016).
- Niwa, S.; Yoshino, S.; Kurosaka, M.; Shino, K.; Yamamoto, S. Reconstruction of the Knee Joint; Springer: Berlin, Germany, 1996. [Google Scholar]
- Per-Ingvar, B.; Zarb, G.A.; Albrektsson, T.; Rosen, H.M. Tissue-Integrated Prostheses: Osseointegration in Clinical Dentistry. J. Prosthet. Dent. 1985, 54, 611–612. [Google Scholar]
- Patil, S.; Luis, C.; Finn, H. Porous Femoral Fixation in Total Hip Arthroplasty with Short Anatomical Stem: Radiographic Evaluation. Clin. Orthop. Surg. 2017, 9, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Laskin, R.S. Total Knee Replacement; Springer: Berlin, Germany, 1991. [Google Scholar]
- Kienapfel, H.; Sprey, C.; Wilke, A.; Griss, R. Implant Fixation by Bone Ingrowth. J. Arthroplast. 1999, 14, 355–368. [Google Scholar] [CrossRef]
- Goodman, S.; Aspenberg, P. Effect of amplitude of micromotion on bone ingrowth into titanium chambers implanted in the rabbit tibia. Biomaterials 1992, 13, 944–948. [Google Scholar] [CrossRef]
- Szmukler-Moncler, S.; Salama, H.; Reingewirtz, Y.; Dubruille, J.H. Timing of Loading and Effect of Micromotion on Bone–Dental Implant Interface: Review of Experimental Literature. J. Biomed. Mater. Res. Appl. Biomater. 1997, 43, 192–203. [Google Scholar] [CrossRef]
- Liu, X.; Niebur, G.L. Bone ingrowth into a porous coated implant predicted by a mechano-regulatory tissue differentiation algorithm. Biomech. Model. Mechanobiol. 2008. [Google Scholar] [CrossRef] [PubMed]
- Jasty, M.; Krushell, R.; Zalenski, E.; O’Connor, D.; Sedlacek, R.; Harris, W. The contribution of the nonporous distal stem to the stability of proximally porous-coated canine femoral components. J. Arthroplast. 1993, 8, 33–41. [Google Scholar] [CrossRef]
- Conlisk, N.; Howie, C.; Pankaj, P. Computational modelling of motion at the bone-implant interface after total knee arthroplasty: The role of implant design and surgical fit. Knee 2017, 24, 994–1005. [Google Scholar] [CrossRef] [PubMed]
- Udofia, I.; Liu, F.; Jin, Z.; Roberts, P.; Grigoris, P. The initial stability and contact mechanics of a press-fit resurfacing arthroplasty of the hip. Bone Jt. J. 2007, 89, 549–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aspenberg, P.; Goodman, S.; Toksvig-Larsen, S.; Ryd, L.; Albrektsson, T. Intermittent micromotion inhibits bone ingrowth. Acta Orthop. Scand. 1992, 63, 141–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawahara, H.; Kawahara, D.; Hayakawa, M.; Tamai, Y.; Kuremoto, T.; Matsuda, S. Osseointegration under immediate loading: Biomechanical stress-strain and bone formation–resorption. Implant Dent. 2003, 12, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Gortchacow, M.; Wettstein, M.; Pioletti, D.P.; Muller-Gerbl, M.; Terrier, A. Simultaneous and multisite measure of micromotion, subsidence and gap to evaluate femoral stem stability. J. Biomech. 2012, 45, 1232–1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilliar, R.; Lee, J.; Maniatopoulos, C. Observations on the effect of movement on bone ingrowth into porous-surfaced implants. Clin. Orthop. Relat. Res. 1986, 208, 108–113. [Google Scholar] [CrossRef]
- Bragdon, J.M.; Burke, C.; O’Connor, D.; Lowenstein, D.; Harris, J. In vivo skeletal responses to porous-surfaced implants subjected to small induced motions. J. Bone Jt. Surg. Am. 1997, 79, 1232–1238. [Google Scholar]
- Karl, M.; Graef, F.; Winter, W. Determination of Micromotion at the Implant Bone Interface—An In-Vitro Methodologic Study. Dentistry 2015, 5, 289. [Google Scholar]
- Varga, M.; Wolter, K.J. Sensors and imaging methods for detecting loosening of orthopedic implants—A review. In Proceedings of the IEEE 20th International Symposium for Design and Technology in Electronic Packaging, Bucharest, Romania, 23–26 October 2014. [Google Scholar]
- Dihlmann, W.; Dihlmann, S.W.; Hering, L. Alloarthroplasty of the hip joint. Radiologic diagnosis of loosening and infection in cemented total endoprostheses. Radiologe 1991, 31, 496–505. [Google Scholar] [PubMed]
- Cahir, J.; Toms, A.; Marshall, T.; Wimhurst, J.; Nolan, J. CT and MRI of hip arthroplasty. Clin. Radiol. 2007, 62, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Cooper, H.J.; Ranawat, A.S.; Potter, H.G.; Foo, L.F.; Jawetz, S.T.; Ranawat, C.S. Magnetic Resonance Imaging in the Diagnosis and Management of Hip Pain After Total Hip Arthroplasty. J. Arthroplast. 2009, 24, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Mayer-Wagner, S.; Mayer, W.; Maegerlein, S.; Linke, R.; Jansson, V.; Müller, P.E. Use of 18F-FDG-PET in the diagnosis of endoprosthetic loosening of knee and hip implants. Arch. Orthop. Trauma Surg. 2010, 130, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Ryd, L. Micromotion in knee arthroplasty. Acta Orthop. Scand. 1986, 57, 3–80. [Google Scholar] [CrossRef] [Green Version]
- Camine, V.M.; Rüdiger, H.; Pioletti, D.; Terrier, A. Full-field measurement of micromotion around a cementless femoral stem using micro-CT imaging and radiopaque markers. J. Biomech. 2016, 49, 4002–4008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgiou, A.P.; Cunningham, J.L. Accurate diagnosis of hip prostehsis loosening using a vibrational techniques. Clin. Biomech. 2001, 16, 315–323. [Google Scholar] [CrossRef]
- DiSilvestro, M.; Swope, S.; Dietz, T.; McNulty, D. The design and development of a measurement system for the investigation of dynamic micromotion in total knee joint replacements. IEEE Trans. Instrum. Meas. 2005, 54, 1126–1132. [Google Scholar] [CrossRef]
- Jauch, S.Y.; Huber, G.; Sellenschloh, K.; Haschke, H.; Baxmann, M.; Grupp, T.M.; Morlock, M.M. Micromotions at the taper interface between stem and neck adapter of a bimodular hip prosthesis during activities of daily living. J. Orthop. Res. 2013, 31, 1165–1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khokle, R.; Esselle, K.; Heimlich, M.; Bokor, D. Orthopaedic implant micromotion sensing using an eddy current sensor. In Proceedings of the 2017 IEEE Life Sciences Conference (LSC), Sydney, Australia, 13–15 December 2017; pp. 125–128. [Google Scholar]
- Wilson, J.S. Sensor Technology Handbook; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Lai, Y. Eddy Current Displacement Sensor with LTCC Technology. Ph.D. Thesis, Albert Ludwigs University of Freiburg, Freiburg im Breisgau, Germany, 2014. [Google Scholar]
- Misakian, M. Equations for the Magnetic Field Produced by One or More Rectangular Loops of Wire in the Same Plane. J. Res. Natl. Inst. Stand. Technol. 2000, 105, 557. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Itakura, H. Input Impedance Characteristics of Small Rectangular Loop Antenna. In Proceedings of the ISAP Sapporo Japan, Sapporo, Japan, 22 September 1992; pp. 673–676. [Google Scholar]
- May, P.; Zhou, E. Numerical Modelling; Chapter Numerical Modelling and Design of an Eddy Current Sensor; InTech, 2012; pp. 159–184. Available online: https://www.intechopen.com/download/pdf/33083 (accessed on 10 July 2018).
- Kuo, L.C.; Chen, W.T.; Chuang, H.R. Numerical computation of human body effects on radiation characteristics of loop antennas for watch-type wrist radiophone application. In Proceedings of the Asia-Pacific Microwave Conference (Cat. No. 01TH8577), Taipei, Taiwan, 3–6 December 2001; pp. 1307–1310. [Google Scholar]
- Kracek, J.; Svanda, M.; Mazanek, M.; Machac, J. Semi-active 866 MHz RFID implantable tag fed by 6.78 MHz inductive wireless power transfer. In Proceedings of the 46th European Microwave Conference (EuMC), London, UK, 4–6 October 2016; pp. 620–622. [Google Scholar]
- Kracek, J.; Švanda, M.; Mazanek, M.; Machac, J. Implantable Semi-Active UHF RFID Tag with Inductive Wireless Power Transfer. IEEE Antennas Wirel. Propag. Lett. 2016, 15, 1657–1660. [Google Scholar] [CrossRef]
- Khokle, R.P.; Esselle, K.P.; Heimlich, M.; Bokor, D. Design of a miniaturized bone implantable antenna for a wireless implant monitoring device. In Proceedings of the Loughborough Antennas Propagation Conference (LAPC 2017), Loughborough, UK, 13–14 November 2017; pp. 1–2. [Google Scholar]







| Australia | United Kingdom | Holland | New Zealand | Portugal | |
|---|---|---|---|---|---|
| Hip Primary | 440,841 | 800,683 | 125,391 | 110,208 | 4384 |
| Hip Revision | 57,819 | 89,023 | 16,991 | 16,251 | 648 |
| Burden % | 11.59 | 10.00 | 11.93 | 12.85 | 12.87 |
| Knee Primary | 544,075 | 875,585 | 116,780 | 95,821 | 4110 |
| Knee Revision | 48,502 | 54,278 | 10,360 | 6739 | 291 |
| Burden % | 8.18 | 5.83 | 8.14 | 6.57 | 6.61 |
| Shoulder Primary | 29,068 | 17,300 | 2077 | 7305 | 111 |
| Shoulder Revision | 3338 | 2045 | 203 | 571 | 9 |
| Burden % | 10.30 | 10.57 | 8.90 | 7.24 | 7.5 |
| Ankle Primary | 1662 | 3185 | 122 | 1261 | 17 |
| Ankle Revision | 376 | 358 | 15 | 179 | 1 |
| Burden % | 18.44 | 10.10 | 10.94 | 12.43 | 5.55 |
| Elbow Primary | 2738 | 1639 | 107 | 476 | 66 |
| Elbow Revision | 536 | 507 | 38 | 81 | 4 |
| Burden % | 16.37 | 23.62 | 26.20 | 14.54 | 5.71 |
| Order | Mesh Elements | RAM | CPU Time (Min:S) | |
|---|---|---|---|---|
| First Order | 8 | 76719 | 4.81 GB | 56:31 |
| Second Order | 4 | 28400 | 7.08 GB | 35:52 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khokle, R.P.; Esselle, K.P.; Bokor, D.J. Design, Modeling, and Evaluation of the Eddy Current Sensor Deeply Implanted in the Human Body. Sensors 2018, 18, 3888. https://doi.org/10.3390/s18113888
Khokle RP, Esselle KP, Bokor DJ. Design, Modeling, and Evaluation of the Eddy Current Sensor Deeply Implanted in the Human Body. Sensors. 2018; 18(11):3888. https://doi.org/10.3390/s18113888
Chicago/Turabian StyleKhokle, Rajas Prakash, Karu P. Esselle, and Desmond J. Bokor. 2018. "Design, Modeling, and Evaluation of the Eddy Current Sensor Deeply Implanted in the Human Body" Sensors 18, no. 11: 3888. https://doi.org/10.3390/s18113888





