Improvement of Detection Sensitivity of Microbubbles as Sensors to Detect Ambient Pressure †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ultrasound Contrast Agent Microbubbles
2.2. Acoustic Attenuation Measurement
2.3. Acoustic Transmission Measurement
2.4. Acoustic Scattering Measurement
3. Results
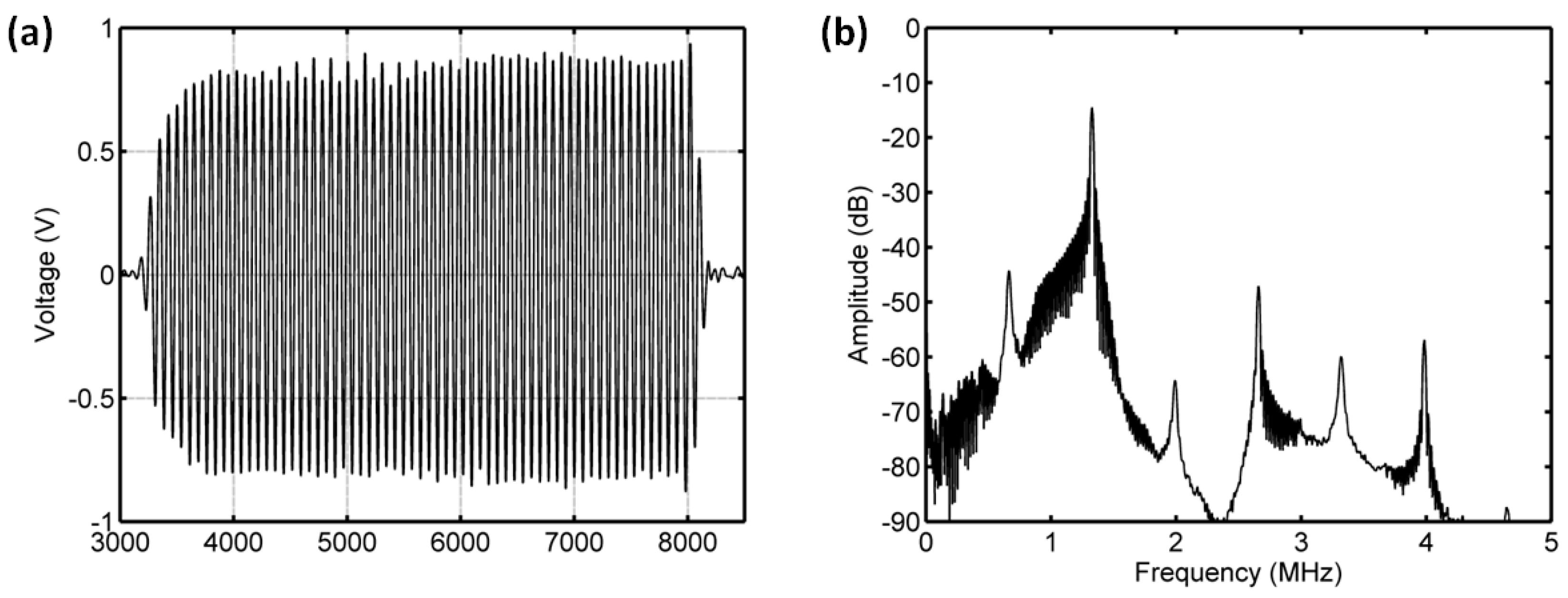
3.1. The Resonance Frequency of UCA Microbubbles
3.2. Effects of Driving Frequency on Subharmonic Scattering Power
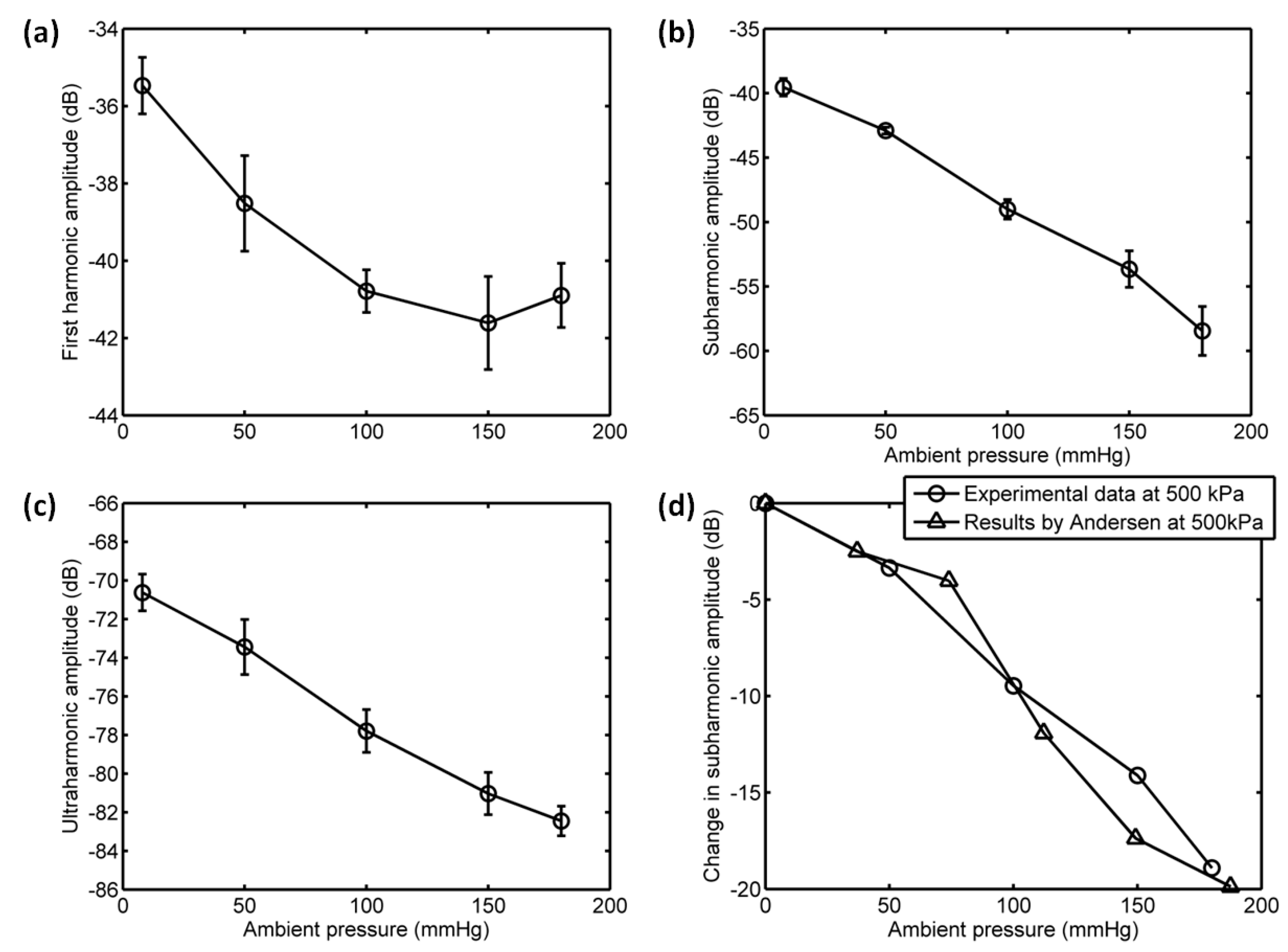
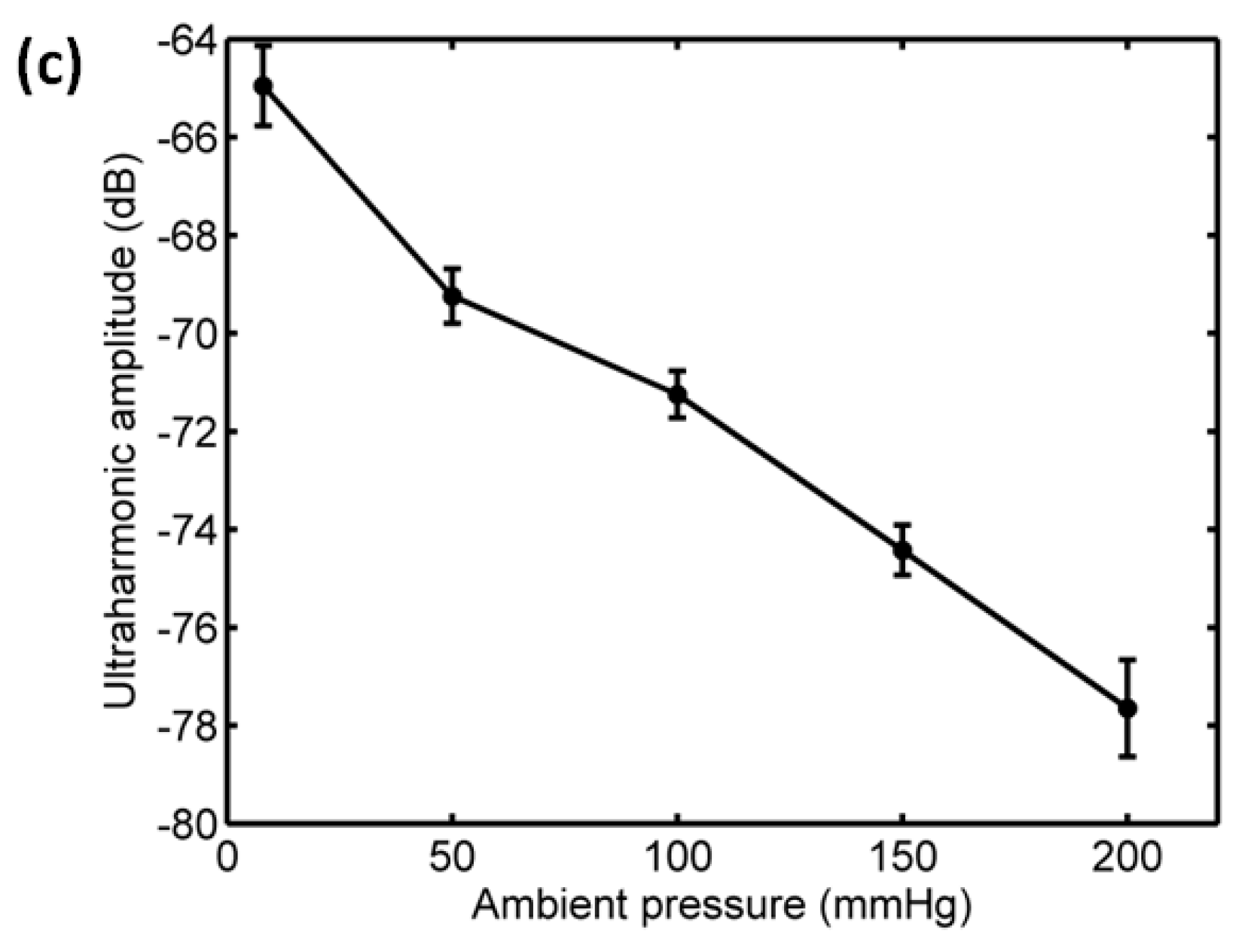
3.3. The Relationship between Scattering Power and Ambient Pressure
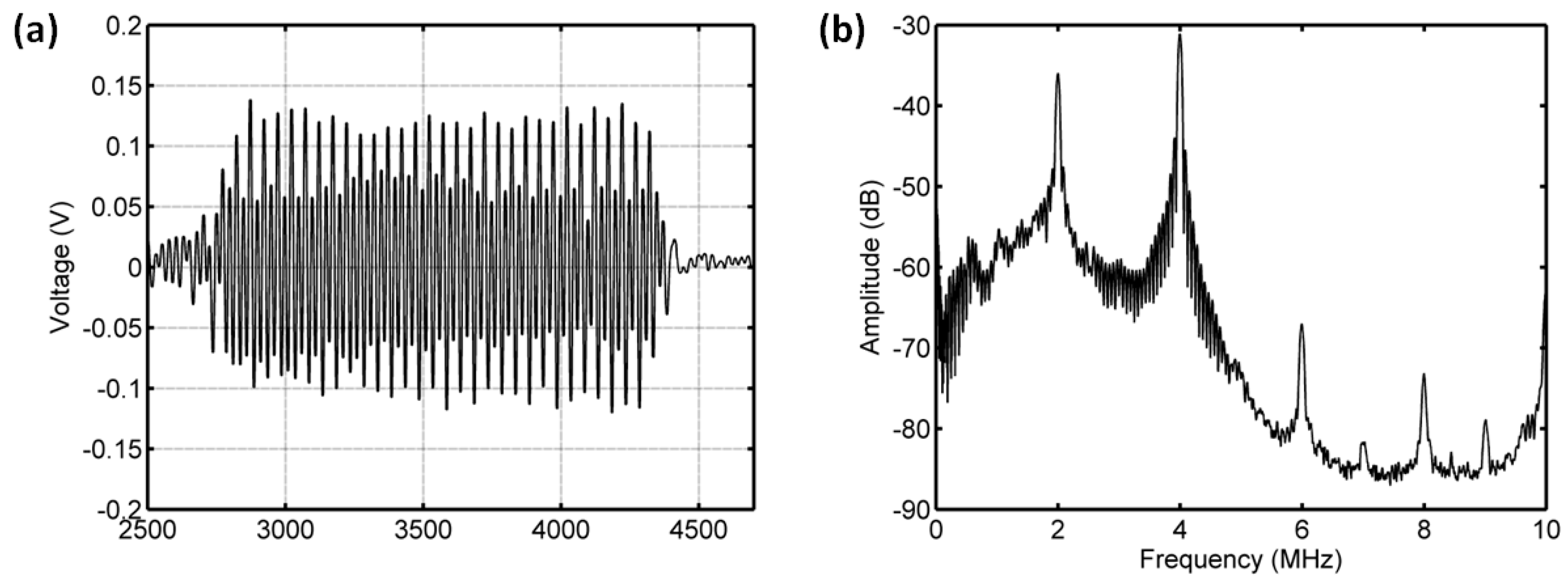
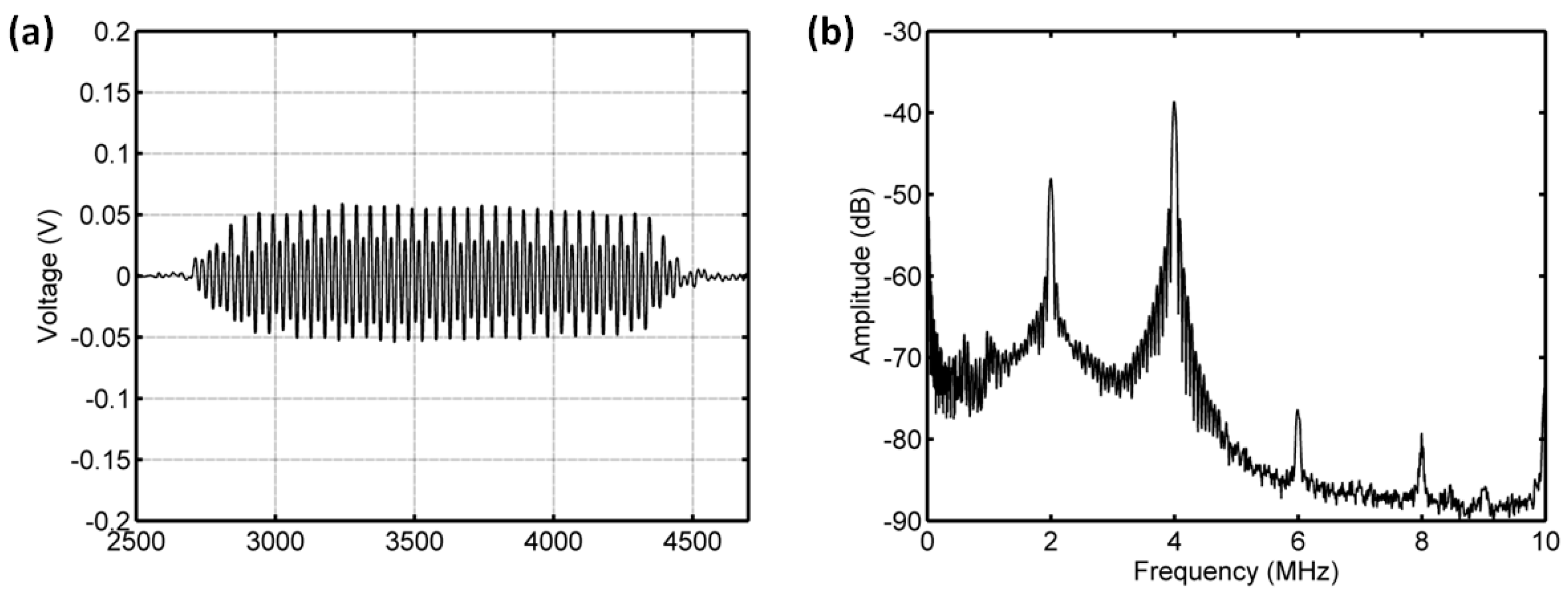
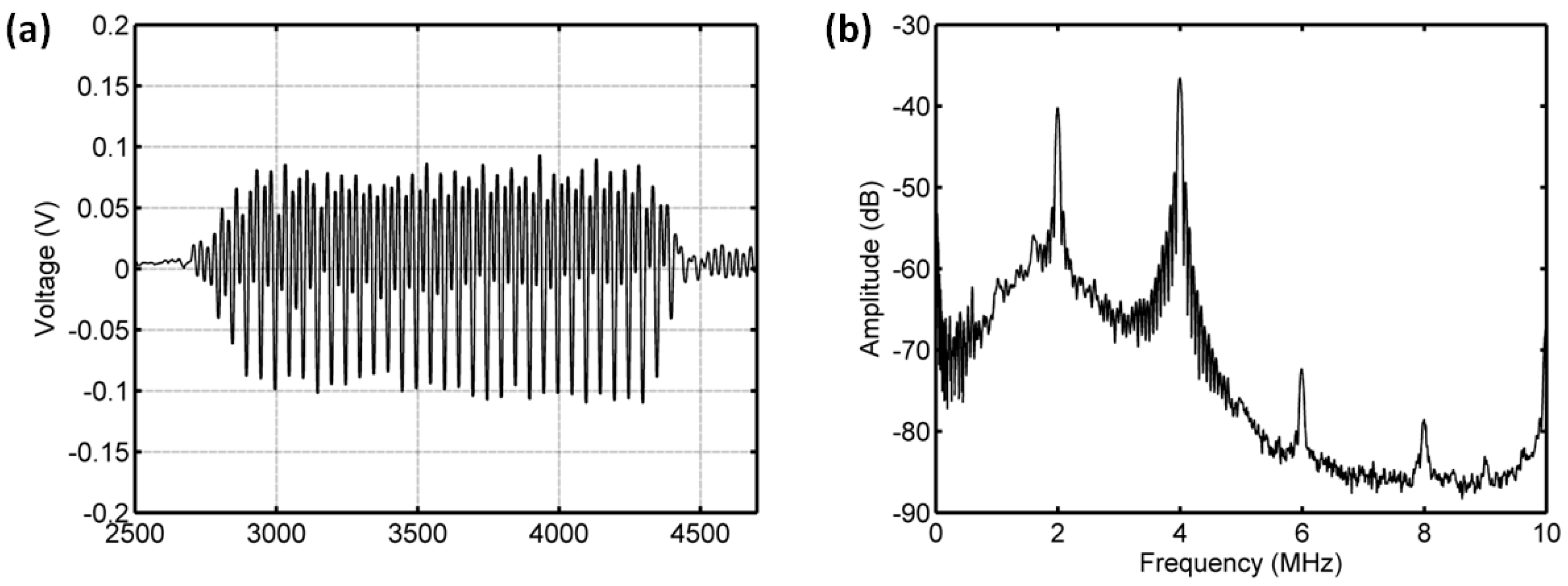
3.3.1. Acoustic Scattering Signals at 0.175 MI
3.3.2. Acoustic Scattering Signals at 0.225 MI
3.3.3. Acoustic Scattering Signals at 0.25 MI
3.3.4. Acoustic Scattering Signals at 0.3 MI
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shi, W.T.; Forsberg, F.; Raichlen, J.S.; Needleman, L.; Goldberg, B.B. Pressure dependence of subharmonic signals from contrast microbubbles. Ultrasound Med. Biol. 1999, 25, 275–283. [Google Scholar] [CrossRef]
- Dave, J.K.; Kulkarni, S.V.; Pangaonkar, P.P.; Stanczak, M.; McDonald, M.E.; Cohen, I.S.; Mehrotra, P.; Savage, M.P.; Walinsky, P.; Ruggiero, N.J., II; et al. Non-invasive intra-cardiac pressure measurements using subharmonic-aided pressure estimation: Proof of concept in humans. Ultrasound Med. Biol. 2017, 43, 2718–2724. [Google Scholar] [CrossRef] [PubMed]
- Andersen, K.S.; Jensen, J.A. Impact of acoustic pressure on ambient pressure estimation using ultrasound contrast agent. Ultrasonics 2010, 50, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Fairbank, W.M.; Scully, M.O. New noninvasive technique for cardiac pressure measurement—Resonant scattering of ultrasound from bubbles. IEEE Trans. Biomed. Eng. 1977, 24, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Bouakaz, A.; Frinking, P.J.A.; de Jong, N.; Bom, N. Noninvasive measurement of the hydrostatic pressure in a fluid-filled cavity based on the disappearance time of micrometer-sized free gas bubbles. Ultrasound Med. Biol. 1999, 25, 1407–1415. [Google Scholar] [CrossRef]
- Postema, M.; Bouakaz, A.; de Jong, N. Noninvasive microbubble-based pressure measurements: A simulation study. Ultrasonics 2004, 42, 759–762. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Kitabatake, A.; Tanouchi, J.; Fujii, K.; Uematsu, M.; Yoshida, Y.; Kamada, T.; Tamura, T.; Chihara, K.; Shirae, K. New approach to noninvasive manometry based on pressure dependent resonant shift of elastic microcapsules in ultrasonic frequency-characteristics. Jpn. J. Appl. Phys. Part 1-Regul. Pap. Short Notes Rev. Pap. 1988, 27, 125–127. [Google Scholar] [CrossRef]
- Oesterle, S.; Sahines, T.; Tucker, C.; Tickner, E.G.; Rasor, J.; Kernoff, R.; Kantrowitz, N.; Billingham, M.; Wagner, L.; Popp, R.L. A new method for assessing right-sided heart pressures using encapsulated microbubbles—A preliminary-report. West. J. Med. 1985, 143, 463–468. [Google Scholar] [PubMed]
- Li, F.; Wang, L.; Fan, Y.; Li, D. Simulation of Noninvasive Blood Pressure Estimation Using Ultrasound Contrast Agent Microbubbles. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2012, 59, 715–726. [Google Scholar] [PubMed]
- Jimenez-Fernandez, J. Dependence of the subharmonic signal from contrast agent microbubbles on ambient pressure: A theoretical analysis. J. Acoust. Soc. Am. 2018, 143, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Ling, T.; Liu, C.; Jin, Q.; Cai, F.; Zheng, H.; Li, D. Ambient pressure dependence of the subharmonic signal from ultrasound contrast microbubbles. In Proceedings of the 2012 IEEE International Ultrasonics Symposium, Dresden, Germany, 7–10 October 2012; pp. 663–666. [Google Scholar]
- Tremblay-Darveau, C.; Williams, R.; Burns, P.N. Measuring absolute blood pressure using microbubbles. Ultrasound Med. Biol. 2014, 40, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Hok, B. A new approach to non-invasive manometry—Interaction between ultrasound and bubbles. Med. Biol. Eng. Comput. 1981, 19, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, J.R.; Reisner, S.A.; Lichtenberg, G.S.; Meltzer, R.S. Intravenous contrast echocardiography with use of sonicated albumin in humans—Systolic disappearance of left-ventricular contrast after transpulmonary transmission. J. Am. Coll. Cardiol. 1990, 16, 1603–1607. [Google Scholar] [CrossRef]
- Dejong, N.; Tencate, F.J.; Vletter, W.B.; Roelandt, J. Quantification of transpulmonary echocontrast effects. Ultrasound Med. Biol. 1993, 19, 279–288. [Google Scholar] [Green Version]
- Gottlieb, S.; Ernst, A.; Meltzer, R.S. Effect of pressure on echocardiographic videodensity from sonicated albumin—An in-vitro model. J. Ultrasound Med. 1995, 14, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Tiukinhoy, S.; Bhoopalam, S.; Winkelmann, J.W.; Ammar, K.; Mangulabnan, L.; Reddy, G.; Fernandez, A.; Block, R.; Malhotra, V.; Feinstein, S. Loss of contrast intensity during systole in the left ventricular cavity with the use of the contrast agent Albunex—An analysis of its correlation with pressure and velocity. Investig. Radiol. 1996, 31, 415–422. [Google Scholar] [CrossRef]
- Brayman, A.A.; Azadniv, M.; Miller, M.W.; Meltzer, R.S. Effect of static pressure on acoustic transmittance of Albunex(R) microbubble suspensions. J. Acoust. Soc. Am. 1996, 99, 2403–2408. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, F.; Liu, J.B.; Shi, W.T.; Furuse, J.; Shimizu, M.; Goldberg, B.B. In vivo pressure estimation using subharmonic contrast microbubble signals: Proof of concept. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2005, 52, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Leodore, L.A.; Forsberg, F.; Shi, W.T. P5B-6 In Vitro Pressure Estimation Obtained from Subharmonic Contrast Microbubble Signals. In Proceedings of the Ultrasonics Symposium, New York, NY, USA, 28–31 October 2007; pp. 2207–2210. [Google Scholar]
- Adam, D.; Sapunar, M.; Burla, E. On the relationship between encapsulated ultrasound contrast agent and pressure. Ultrasound Med. Biol. 2005, 31, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Dave, J.K.; Halldorsdottir, V.G.; Eisenbrey, J.R.; Liu, J.-B.; McDonald, M.E.; Dickie, K.; Leung, C.; Forsberg, F. Noninvasive Estimation of Dynamic Pressures In Vitro and In Vivo Using the Subharmonic Response from Microbubbles. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2011, 58, 2056–2066. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Jia, N.; Zhang, D.; Xu, D. Ambient pressure dependence of the ultra-harmonic response from contrast microbubbles. J. Acoust. Soc. Am. 2012, 131, 4358–4364. [Google Scholar] [CrossRef] [PubMed]
- Frinking, P.J.A.; Gaud, E.; Brochot, J.; Arditi, M. Subharmonic Scattering of Phospholipid-Shell Microbubbles at Low Acoustic Pressure Amplitudes. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2010, 57, 1762–1771. [Google Scholar] [CrossRef] [PubMed]
- Andersen, K.S.; Jensen, J.A. Ambient pressure sensitivity of microbubbles investigated through a parameter study. J. Acoust. Soc. Am. 2009, 126, 3350–3358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katiyar, A.; Sarkar, K.; Forsberg, F. Modeling subharmonic response from contrast microbubbles as a function of ambient static pressure. J. Acoust. Soc. Am. 2011, 129, 2325–2335. [Google Scholar] [CrossRef] [PubMed]
- Halldorsdottir, V.G.; Dave, J.K.; Marshall, A.; Forsberg, A.I.; Fox, T.B.; Eisenbrey, J.R.; Machado, P.; Liu, J.-B.; Merton, D.A.; Forsberg, F. Subharmonic-aided pressure estimation for monitoring interstitial fluid pressure in tumors: Calibration and treatment with paclitaxel in breast cancer xenografts. Ultrasound Med. Biol. 2017, 43, 1401–1410. [Google Scholar] [CrossRef] [PubMed]
- Halldorsdottir, V.G.; Dave, J.K.; Eisenbrey, J.R.; Machado, P.; Zhao, H.; Liu, J.B.; Merton, D.A.; Forsberg, F. Subharmonic aided pressure estimation for monitoring interstitial fluid pressure in tumours—In vitro and in vivo proof of concept. Ultrasonics 2014, 54, 1938–1944. [Google Scholar] [CrossRef] [PubMed]
- Halldorsdottir, V.G.; Dave, J.K.; Leodore, L.M.; Eisenbrey, J.R.; Park, S.; Hall, A.L.; Thomeniuv, K.; Forsberg, F. Subharmonic Contrast Microbubble Signals for Noninvasive Pressure Estimation under Static and Dynamic Flow Conditions. Ultrason. Imaging 2011, 33, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.; Eisenbrey, J.R.; Stanczak, M.; Sridharan, A.; Berger, A.C.; Avery, T.; Palazzo, J.P.; Forsberg, F. Monitoring Neoadjuvant Chemotherapy for Breast Cancer by Using Three-dimensional Subharmonic Aided Pressure Estimation and Imaging with US Contrast Agents: Preliminary Experience. Radiology 2017, 285, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Gupta, I.; Eisenbrey, J.; Stanczak, M.; Sridharan, A.; Dave, J.K.; Liu, J.-B.; Hazard, C.; Wang, X.; Wang, P.; Li, H.; et al. Effect of Pulse Shaping on Subharmonic Aided Pressure Estimation In Vitro and In Vivo. J. Ultrasound Med. 2017, 36, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Dave, J.K.; Halldorsdottir, V.G.; Eisenbrey, J.R.; Merton, D.A.; Liu, J.B.; Machado, P.; Zhao, H.; Park, S.; Dianis, S.; Chalek, C.L.; et al. On the implementation of an automated acoustic output optimization algorithm for subharmonic aided pressure estimation. Ultrasonics 2013, 53, 880–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dave, J.K.; Halldorsdottir, V.G.; Eisenbrey, J.R.; Forsberg, F. Processing of Subharmonic Signals from Ultrasound Contrast Agents to Determine Ambient Pressures. Ultrason. Imaging 2012, 34, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Eisenbrey, J.R.; Dave, J.K.; Halldorsdottir, V.G.; Merton, D.A.; Miller, C.; Gonzalez, J.M.; Machado, P.; Park, S.; Dianis, S.; Chalek, C.L.; et al. Chronic Liver Disease: Noninvasive Subharmonic Aided Pressure Estimation of Hepatic Venous Pressure Gradient. Radiology 2013, 268, 581–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoff, L.; Sontum, P.C.; Hovem, J.M. Oscillations of polymeric microbubbles: Effect of the encapsulating shell. J. Acoust. Soc. Am. 2000, 107, 2272–2280. [Google Scholar] [CrossRef] [PubMed]
- Frinking, P.J.A.; de Jong, N. Acoustic modeling of shell-encapsulated gas bubbles. Ultrasound Med. Biol. 1998, 24, 523–533. [Google Scholar] [CrossRef]
- Shankar, P.M.; Krishna, P.D.; Newhouse, V.L. Subharmonic backscattering from ultrasound contrast agents. J. Acoust. Soc. Am. 1999, 106, 2104–2110. [Google Scholar] [CrossRef] [PubMed]
- Chomas, J.; Dayton, P.; May, D.; Ferrara, K. Nondestructive subharmonic imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2002, 49, 883–892. [Google Scholar] [CrossRef] [PubMed]
- van der Meer, S.M.; Versluis, M.; Lohse, D.; Chin, C.T.; Bouakaz, A.; de Jong, N. The resonance frequency of SonoVue (TM). In Proceedings of the 2004 IEEE Ultrasonics Symposium, Montreal, QC, Canada, 23–27 August 2004; Yuhas, M.P., Ed.; IEEE: Piscataway, NJ, USA, 2004; pp. 343–345. [Google Scholar]
- Chomas, J.E.; Dayton, P.; Allen, J.; Morgan, K.; Ferrara, K.W. Mechanisms of contrast agent destruction. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2001, 48, 232–248. [Google Scholar] [CrossRef] [PubMed]
- Borden, M.A.; Kruse, D.E.; Caskey, C.F.; Zhao, S.K.; Dayton, P.A.; Ferrara, K.W. Influence of lipid shell physicochemical properties on ultrasound-induced microbubble destruction. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2005, 52, 1992–2002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorce, J.M.; Arditi, M.; Schneider, M. Influence of bubble size distribution on the echogenicity of ultrasound contrast agents—A study of SonoVue (TM). Investig. Radiol. 2000, 35, 661–671. [Google Scholar] [CrossRef]
- Mannaris, C.; Averkiou, M.A. Investigation of microbubble response to long pulses used in ultrasound-enhanced drug delivery. Ultrasound Med. Biol. 2012, 38, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Memoli, G.; Baxter, K.O.; Jones, H.G.; Mingard, K.P.; Zeqiri, B. Acoustofluidic Measurements on Polymer-Coated Microbubbles: Primary and Secondary Bjerknes Forces. Micromachines 2018, 9, 404. [Google Scholar] [CrossRef] [PubMed]















| Pa (kPa) | f (MHz) | MI | First harmonic (dB) | Sensitivity (mmHg/dB) | Correlation |
|---|---|---|---|---|---|
| 350 | 4 | 0.175 | 5.0 | −36 | 0.9198 |
| 450 | 4 | 0.225 | 6.57 | −27.4 | 0.8825 |
| 500 | 4 | 0.25 | 4.58 | −39.3 | 0.809 |
| 350 | 1.33 | 0.30 | 18.53 | −9.71 | 0.9503 |
| Pa (kPa) | f (MHz) | MI | Subharmonic (dB) | Sensitivity (mmHg/dB) | Correlation |
|---|---|---|---|---|---|
| 350 | 4 | 0.175 | 2.6 | −67.98 | 0.5322 |
| 450 | 4 | 0.225 | 10.9 | −16.44 | 0.8285 |
| 500 | 4 | 0.25 | 19.8 | −9.1 | 0.9919 |
| 350 | 1.33 | 0.30 | 17.6 | −10.21 | 0.9633 |
| Pa (kPa) | f (MHz) | MI | Ultraharmonic (dB) | Sensitivity (mmHg/dB) | Correlation |
|---|---|---|---|---|---|
| 350 | 4 | 0.175 | 4.22 | −50.44 | 0.7437 |
| 450 | 4 | 0.225 | 9.28 | −19.4 | 0.8232 |
| 500 | 4 | 0.25 | 12.28 | −14.66 | 0.9934 |
| 350 | 1.33 | 0.30 | 12.55 | −14.34 | 0.9846 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Li, D.; Yan, F. Improvement of Detection Sensitivity of Microbubbles as Sensors to Detect Ambient Pressure. Sensors 2018, 18, 4083. https://doi.org/10.3390/s18124083
Li F, Li D, Yan F. Improvement of Detection Sensitivity of Microbubbles as Sensors to Detect Ambient Pressure. Sensors. 2018; 18(12):4083. https://doi.org/10.3390/s18124083
Chicago/Turabian StyleLi, Fei, Deyu Li, and Fei Yan. 2018. "Improvement of Detection Sensitivity of Microbubbles as Sensors to Detect Ambient Pressure" Sensors 18, no. 12: 4083. https://doi.org/10.3390/s18124083




