An Implantable Sensorized Lead for Continuous Monitoring of Cardiac Apex Rotation
Abstract
:1. Introduction
2. Materials and Methods
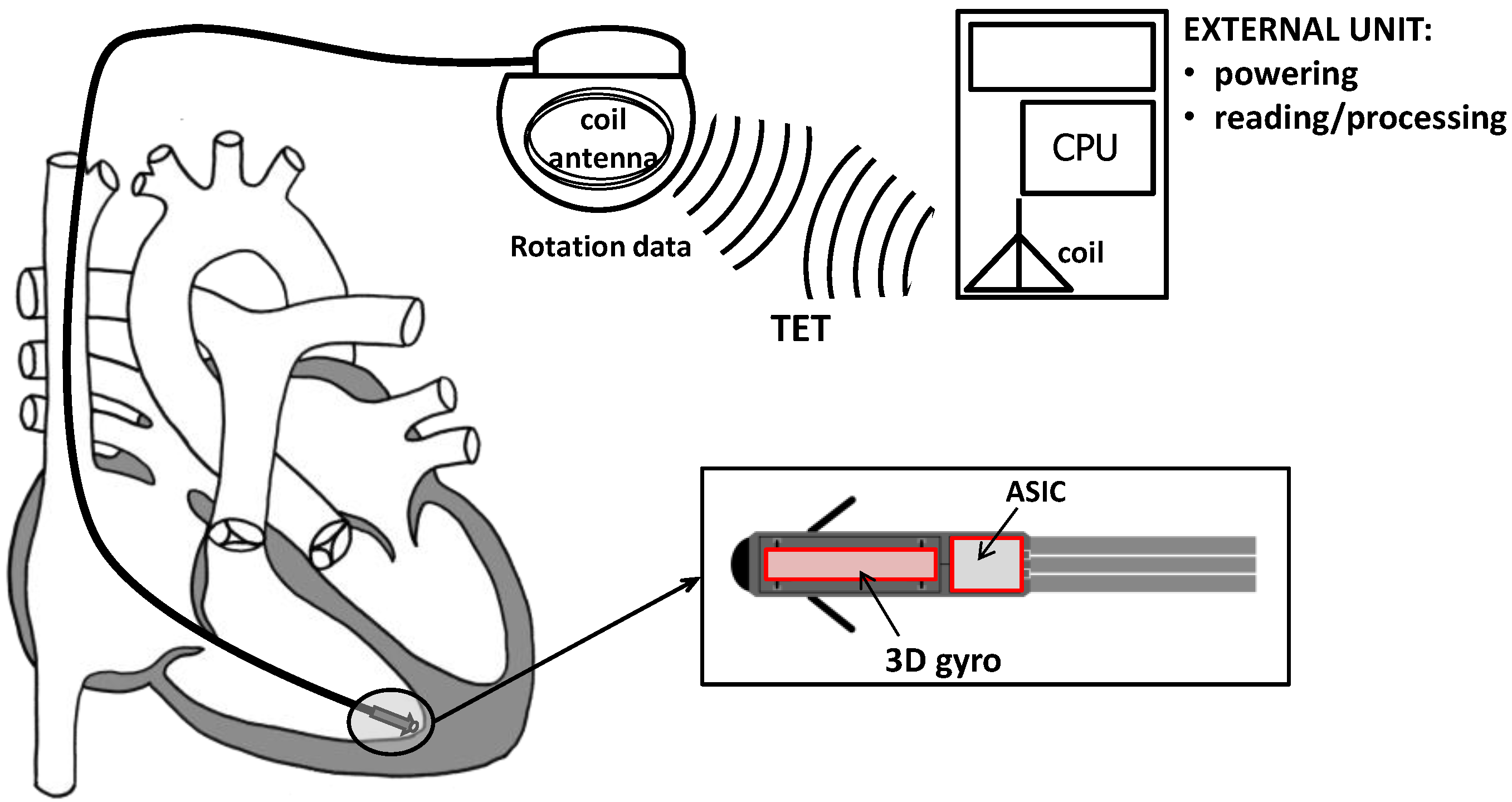
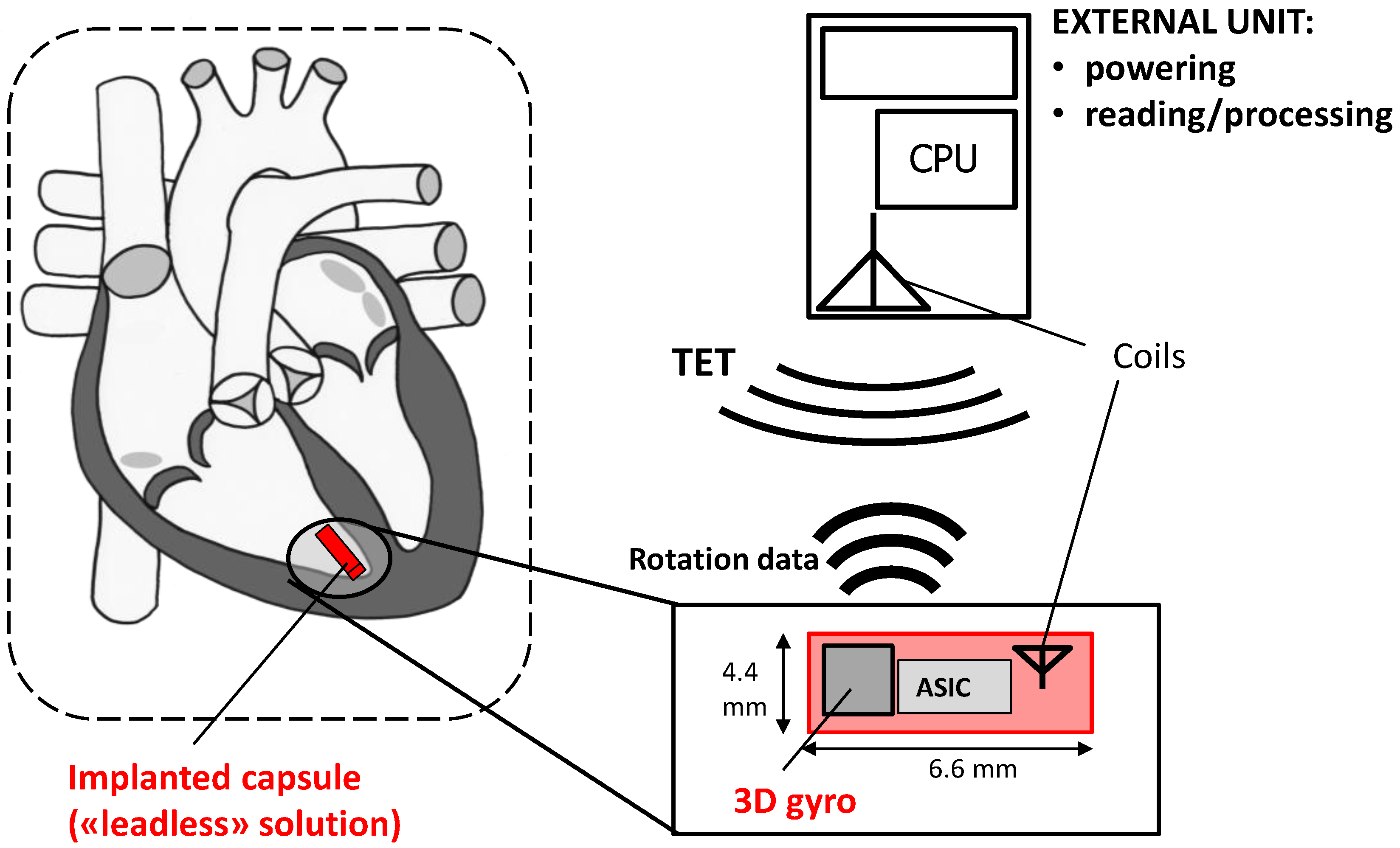
2.1. The Conceptual Design
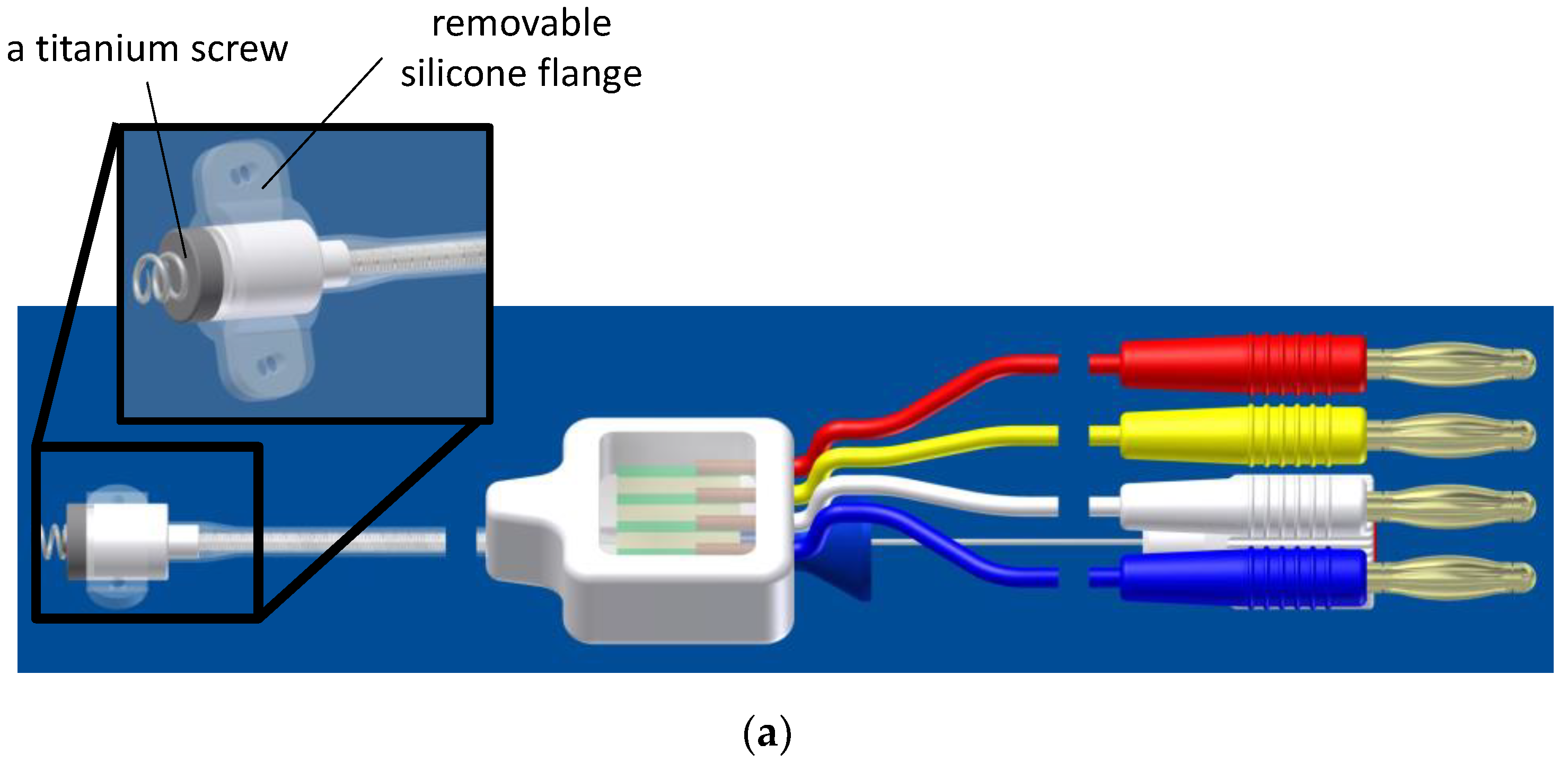
2.2. CardioMon Lead Assembly
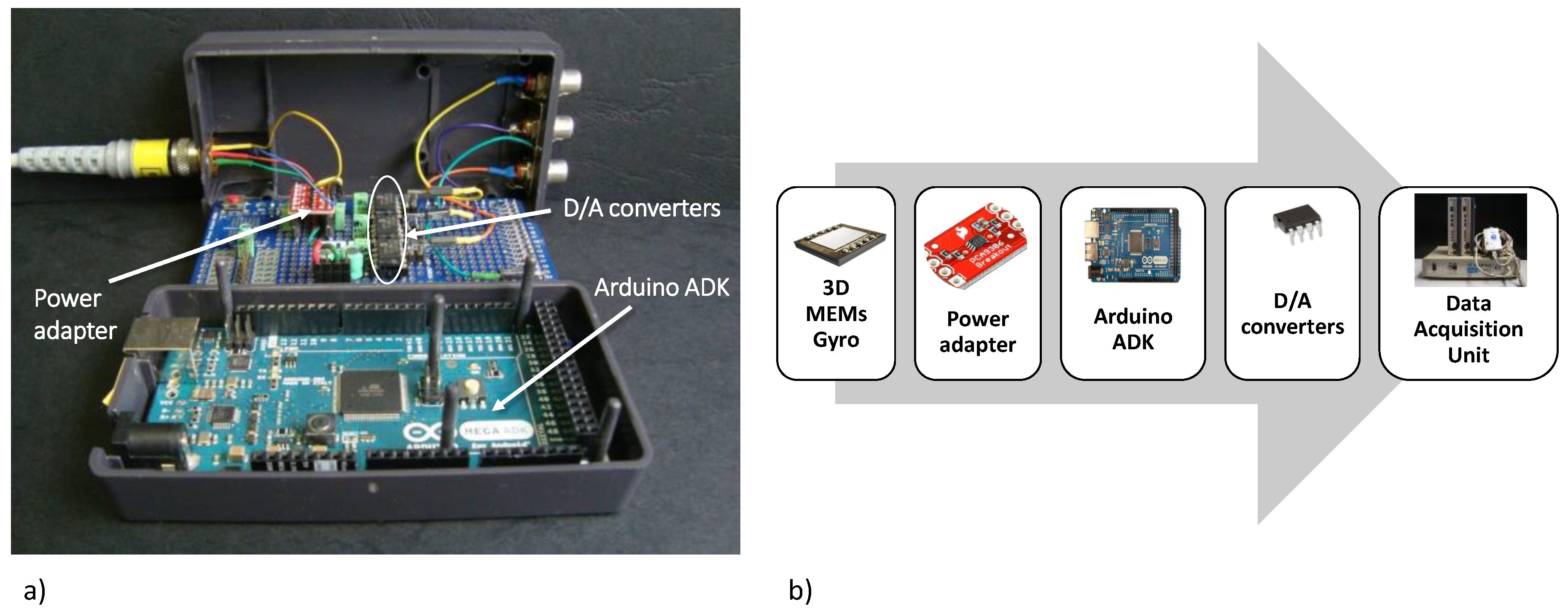
2.3. Data Reading Unit
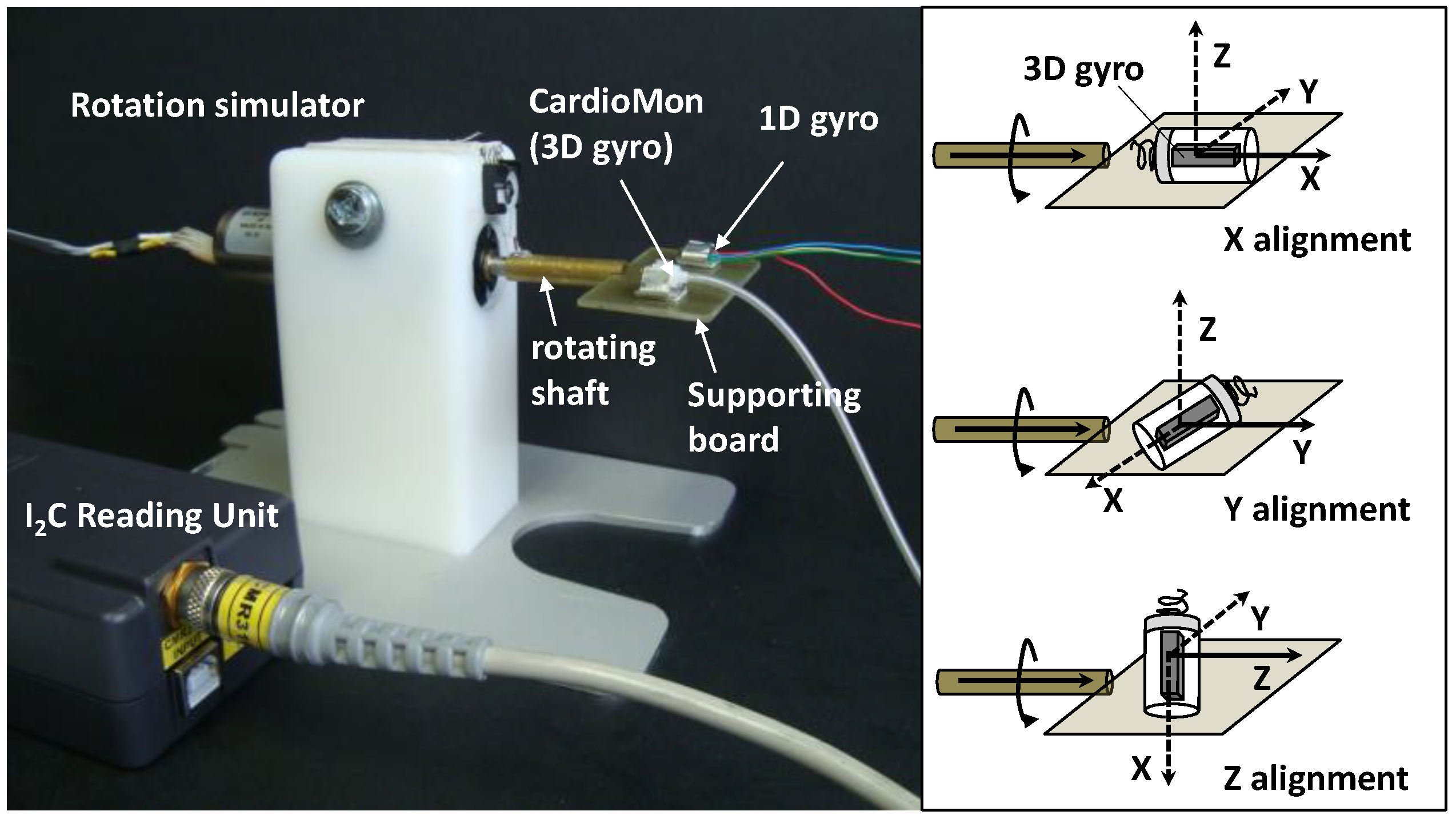
2.4. Functional Lab Tests
2.5. Data Analysis and Statistics
3. Results
4. Discussion
5. Future Perspective for CardioMon Implantation in Humans
Author Contributions
Funding
Conflicts of Interest
References
- Beladan, C.C.; Călin, A.; Roşca, M.; Ginghină, C.; Popescu, B.A. Left ventricular twist dynamics: Principles and applications. Heart Br. Card. Soc. 2014, 100, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, E.; Müller, M.F.; Oswald, H.; Thöny, H.; Mohacsi, P.; Hess, O.M. Cardiac rotation and relaxation in patients with chronic heart failure. Eur. J. Heart Fail. 2004, 6, 715–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart Disease and Stroke Statistics-2018 Update: A Report from the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef] [PubMed]
- Ingels, N.B.; Daughters, G.T.; Stinson, E.B.; Alderman, E.L. Measurement of midwall myocardial dynamics in intact man by radiography of surgically implanted markers. Circulation 1975, 52, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.E.; Daughters, G.T., 2nd; Alderman, E.L.; Stinson, E.B.; Baldwin, J.C.; Miller, D.C. Effect of acute human cardiac allograft rejection on left ventricular systolic torsion and diastolic recoil measured by intramyocardial markers. Circulation 1987, 76, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Gibbons Kroeker, C.A.; Ter Keurs, H.E.; Knudtson, M.L.; Tyberg, J.V.; Beyar, R. An optical device to measure the dynamics of apex rotation of the left ventricle. Am. J. Physiol. 1993, 265, H1444–H1449. [Google Scholar] [CrossRef] [PubMed]
- Castro, P.F.; Concepción, R.; Bourge, R.C.; Martı́nez, A.; Alcaino, M.; Deck, C.; Ferrada, M.; Alfaro, M.; Perrone, S. A wireless pressure sensor for monitoring pulmonary artery pressure in advanced heart failure: Initial experience. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2007, 26, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Verdejo, H.E.; Castro, P.F.; Concepción, R.; Ferrada, M.A.; Alfaro, M.A.; Alcaíno, M.E.; Deck, C.C.; Bourge, R.C. Comparison of a radiofrequency-based wireless pressure sensor to swan-ganz catheter and echocardiography for ambulatory assessment of pulmonary artery pressure in heart failure. J. Am. Coll. Cardiol. 2007, 50, 2375–2382. [Google Scholar] [CrossRef] [PubMed]
- Abraham, W.T.; Adamson, P.B.; Bourge, R.C.; Aaron, M.F.; Costanzo, M.R.; Stevenson, L.W.; Strickland, W.; Neelagaru, S.; Raval, N.; Krueger, S.; et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: A randomised controlled trial. Lancet Lond. Engl. 2011, 377, 658–666. [Google Scholar] [CrossRef]
- Abraham, W.T.; Perl, L. Implantable Hemodynamic Monitoring for Heart Failure Patients. J. Am. Coll. Cardiol. 2017, 70, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Ritzema, J.; Melton, I.C.; Richards, A.M.; Crozier, I.G.; Frampton, C.; Doughty, R.N.; Whiting, J.; Kar, S.; Eigler, N.; Krum, H.; et al. Direct left atrial pressure monitoring in ambulatory heart failure patients: Initial experience with a new permanent implantable device. Circulation 2007, 116, 2952–2959. [Google Scholar] [CrossRef] [PubMed]
- Troughton, R.W.; Ritzema, J.; Eigler, N.L.; Melton, I.C.; Krum, H.; Adamson, P.B.; Kar, S.; Shah, P.K.; Whiting, J.S.; Heywood, J.T.; et al. Direct left atrial pressure monitoring in severe heart failure: Long-term sensor performance. J. Cardiovasc. Transl. Res. 2011, 4, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Hubbert, L.; Baranowski, J.; Delshad, B.; Ahn, H. Left Atrial Pressure Monitoring with an Implantable Wireless Pressure Sensor After Implantation of a Left Ventricular Assist Device. ASAIO J. Am. Soc. Artif. Intern. Organs 1992 2017, 63, e60–e65. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Assadsangabi, B.; Hsiang, Y.; Takahata, K. Enabling Angioplasty-Ready “Smart” Stents to Detect In-Stent Restenosis and Occlusion. Adv. Sci. Weinh. Baden-Wurtt. Ger. 2018, 5, 1700560. [Google Scholar] [CrossRef] [PubMed]
- Chow, E.Y.; Beier, B.L.; Francino, A.; Chappell, W.J.; Irazoqui, P.P. Toward an implantable wireless cardiac monitoring platform integrated with an FDA-approved cardiovascular stent. J. Interv. Cardiol. 2009, 22, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Halvorsen, P.; Remme, E.; Espinoza, A.; Hoff, L.; Skulstad, H.; Edvardsen, T.; Fosse, E. Automatic real-time detection of myocardial ischemia by epicardial accelerometer. J. Thorac. Cardiovasc. Surg. 2010, 139, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Halvorsen, P.S.; Espinoza, A.; Fleischer, L.A.; Elle, O.J.; Hoff, L.; Lundblad, R.; Skulstad, H.; Edvardsen, T.; Ihlen, H.; Fosse, E. Feasibility of a three-axis epicardial accelerometer in detecting myocardial ischemia in cardiac surgical patients. J. Thorac. Cardiovasc. Surg. 2008, 136, 1496–1502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcelli, E.; Plicchi, G.; Cercenelli, L.; Bortolami, F. First experimental evaluation of cardiac apex rotation with an epicardial coriolis force sensor. ASAIO J. Am. Soc. Artif. Intern. Organs 1992 2005, 51, 696–701. [Google Scholar] [CrossRef]
- Marcelli, E.; Cercenelli, L.; Plicchi, G.; Auricchio, A. Evaluation of an Innovative Sensor for Cardiac Apex Rotation Monitoring. Int. J. Artif. Organs 2010, 33, 433. [Google Scholar]
- Marcelli, E.; Cercenelli, L.; Parlapiano, M.; Fumero, R.; Bagnoli, P.; Costantino, M.L.; Plicchi, G. Effect of right ventricular pacing on cardiac apex rotation assessed by a gyroscopic sensor. ASAIO J. Am. Soc. Artif. Intern. Organs 1992 2007, 53, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Cercenelli, L.; Marcelli, E. Cardiac apex rotation assessed by an implantable gyro sensor: Correlation with a lv pressure-derived myocardial performance index in experimentally induced ischemia. J. Mech. Med. Biol. 2015, 15, 1540013. [Google Scholar] [CrossRef]
- Marcelli, E.; Cercenelli, L.; Musaico, M.; Bagnoli, P.; Costantino, M.L.; Fumero, R.; Plicchi, G. Assessment of cardiac rotation by means of gyroscopic sensors. In Proceedings of the Computers in Cardiology, Bologna, Italy, 14–17 September 2008; Volume 35, pp. 389–392. [Google Scholar]
- Marcelli, E.; Cercenelli, L.; Parlapiano, M.N.; Gianfranchi, L.; Plicchi, G. A Novel Implantable Sensor to Monitor Both Apical Rotation and Cardiac Phases. Int. J. Artif. Organs 2011, 34, 658. [Google Scholar]
- Cutrì, E.; Bagnoli, P.; Marcelli, E.; Biondi, F.; Cercenelli, L.; Costantino, M.L.; Plicchi, G.; Fumero, R. A mechanical simulator of cardiac wall kinematics. ASAIO J. Am. Soc. Artif. Intern. Organs 1992 2010, 56, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Bagnoli, P.; Malagutti, N.; Gastaldi, D.; Marcelli, E.; Lui, E.; Cercenelli, L.; Costantino, M.L.; Plicchi, G.; Fumero, R. Computational finite element model of cardiac torsion. Int. J. Artif. Organs 2011, 34, 44–53. [Google Scholar] [CrossRef] [PubMed]


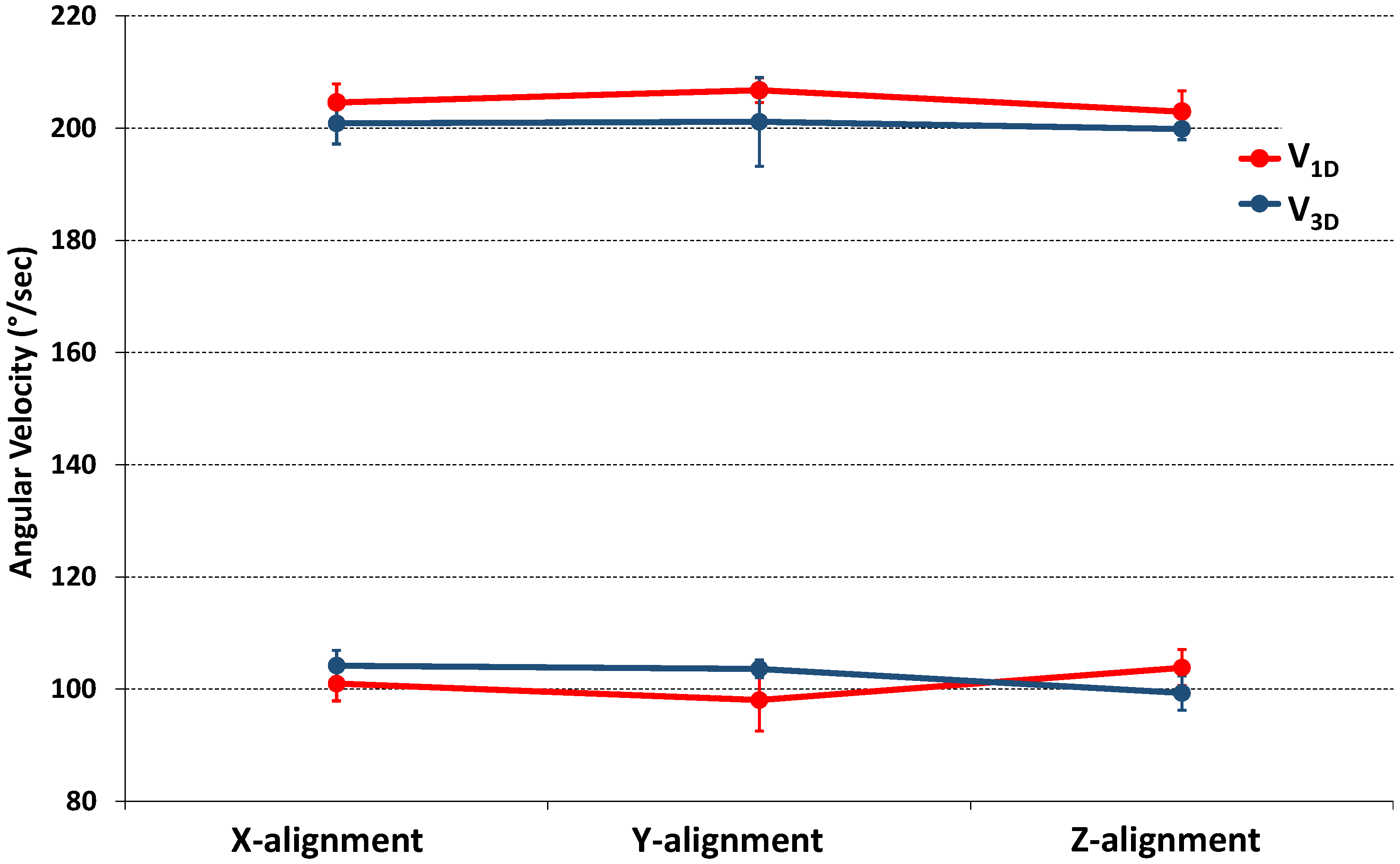
| VT [°/s] | V3D [°/s] | V1D [°/s] | p | |
|---|---|---|---|---|
| X-alig | |VT+| = 200 | 201 ± 4 | 205 ± 3 | 0.218 |
| |VT−| = 100 | 104 ± 3 | 101 ± 3 | 0.065 | |
| Y-alig | |VT+| = 200 | 201 ± 8 | 207 ± 2 | 0.274 |
| |VT−| = 100 | 104 ± 2 | 98 ± 5 | 0.071 | |
| Z-alig | |VT+| = 200 | 199 ± 2 | 203 ± 4 | 0.140 |
| |VT−| = 100 | 99 ± 3 | 104 ± 3 | 0.171 |
| Target | 3D MEMS Gyro | 1D Gyro | |||||
|---|---|---|---|---|---|---|---|
| VT [°/s] | V3D [°/s] | E3D [°/s] | N3D [°/s] | V1D [°/s] | E1D [°/s] | N1D [°/s] | |
| X-alig | V0 = 0 | 7 ± 1 | 8 ± 1 | ||||
| |VT+| = 200 | 201 ± 4 | 4 ± 2 | 205 ± 3 | 4 ± 3 | |||
| |VT−| = 100 | 104 ± 3 | 101 ± 3 | |||||
| Y-alig | V0 = 0 | 7 ± 1 | 8 ± 1 | ||||
| |VT+| = 200 | 201 ± 8 | 5 ± 4 | 207 ± 2 | 6 ± 3 | |||
| |VT−| = 100 | 104 ± 2 | 98 ± 5 | |||||
| Z-alig | V0 = 0 | 7 ± 0 | 10 ± 2 | ||||
| |VT+| = 200 | 199 ± 2 | 2 ± 1 | 203 ± 4 | 4 ± 3 | |||
| |VT−| = 100 | 99 ± 3 | 104 ± 3 | |||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcelli, E.; Cercenelli, L. An Implantable Sensorized Lead for Continuous Monitoring of Cardiac Apex Rotation. Sensors 2018, 18, 4195. https://doi.org/10.3390/s18124195
Marcelli E, Cercenelli L. An Implantable Sensorized Lead for Continuous Monitoring of Cardiac Apex Rotation. Sensors. 2018; 18(12):4195. https://doi.org/10.3390/s18124195
Chicago/Turabian StyleMarcelli, Emanuela, and Laura Cercenelli. 2018. "An Implantable Sensorized Lead for Continuous Monitoring of Cardiac Apex Rotation" Sensors 18, no. 12: 4195. https://doi.org/10.3390/s18124195






