An SPR Sensor Chip Based on Peptide-Modified Single-Walled Carbon Nanotubes with Enhanced Sensitivity and Selectivity in the Detection of 2,4,6-Trinitrotoluene Explosives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Fabrication Procedure of the Peptide-Functionalized SPR Sensor Chip Surface
3. Results and Discussion
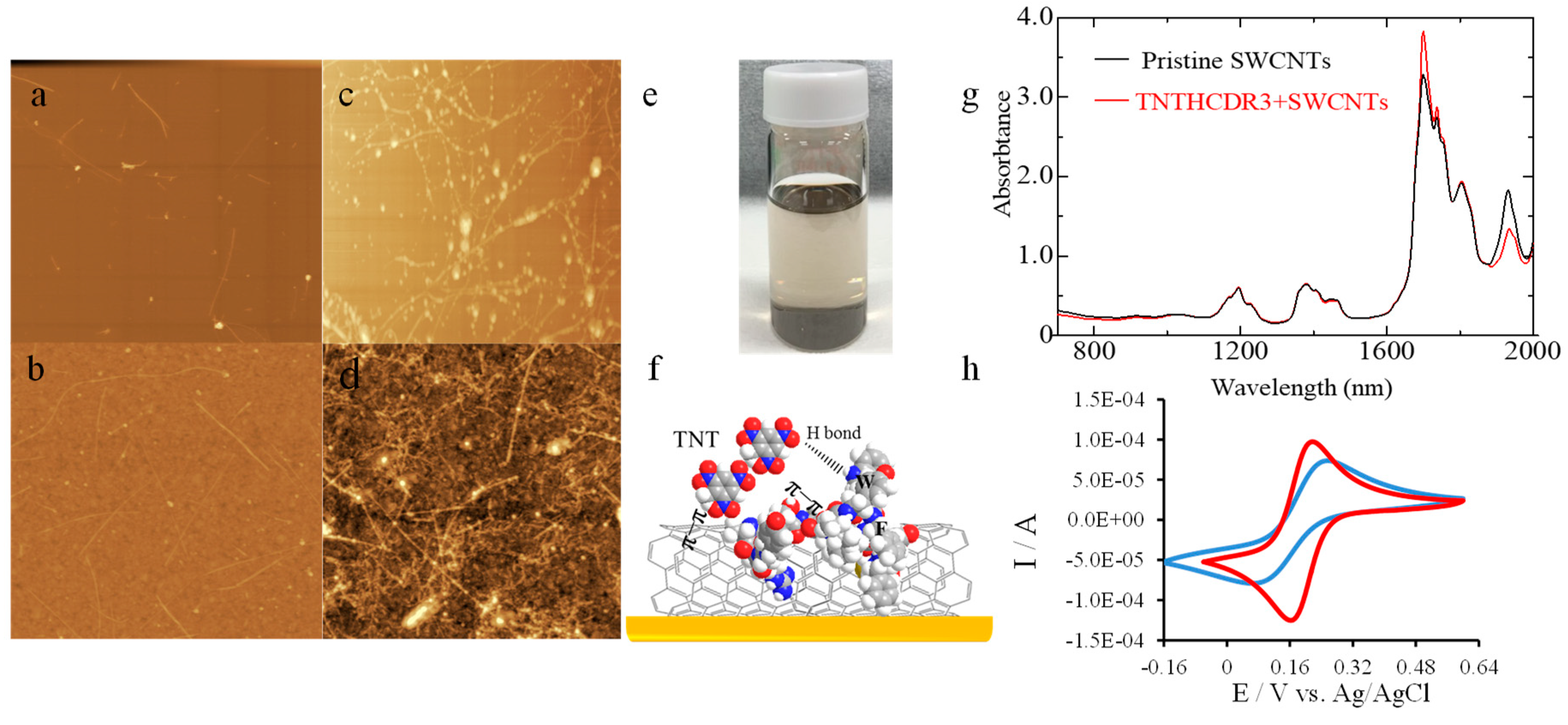
3.1. TNT Recognition Peptide (TNTHCDR3) and SWCNT-Modified SPR Sensor Chip
3.2. Performance of the Peptide–SWCNT-Based SPR Sensor
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Singh, M.; Holzinger, M.; Tabrizian, M.; Winters, S.; Berner, N.C.; Cosnier, S.; Duesberg, G.S. Noncovalently functionalized monolayer graphene for sensitivity enhancement of surface plasmon resonance immunosensors. J. Am. Chem. Soc. 2015, 137, 2800–2803. [Google Scholar] [CrossRef]
- Arsenin, A.V.; Stebunov, Y.V. Biological Sensor and a Method of the Production of Biological Sensor. U.S. Patent 2015/0301039 A1, 22 October 2015. [Google Scholar]
- Jang, D.; Na, W.; Kang, M.; Kim, N.; Shin, S. Ultrasensitive Detection of Single-Walled Carbon Nanotubes Using Surface Plasmon Resonance. Anal. Chem. 2016, 88, 968–973. [Google Scholar] [CrossRef] [PubMed]
- Karajanagi, S.S.; Vertegel, A.A.; Kane, R.S.; Dordick, J.S. Structure and function of enzymes adsorbed onto single-walled carbon nanotubes. Langmuir 2004, 20, 11594–11599. [Google Scholar] [CrossRef]
- Mech, L.D. Immunization with Peptide-Functionalized Carbon Nanotubes Enhances Virus-Specific Neutralizing Antibody Responses. Chem. Biol. 2003, 10, 961–966. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.; Oliver, R.; Li, J.; Lee, J.; Aguilar, M.; Wu, Y. Sensitivity enhancement of SPR assay of progesterone based on mixed self-assembled monolayers using nanogold particles. Biosens. Bioelectron. 2007, 23, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Ghrera, A.S.; Pandey, M.K.; Malhotra, B.D. Quantum dot monolayer for surface plasmon resonance signal enhancement and DNA hybridization detection. Biosens. Bioelectron. 2016, 80, 477–482. [Google Scholar] [CrossRef]
- Wu, L.; Chu, H.S.; Koh, W.S.; Li, E.P. Highly sensitive graphene biosensors based on surface plasmon resonance. Opt. Express 2010, 18, 14395–14400. [Google Scholar] [CrossRef]
- Stebunov, Y.V.; Aftenieva, O.A.; Arsenin, A.V.; Volkov, V.S. Highly Sensitive and Selective Sensor Chips with Graphene-Oxide Linking Layer. ACS Appl. Mater. Interfaces 2015, 7, 21727–21734. [Google Scholar] [CrossRef]
- Okochi, M.; Muto, M.; Yanai, K.; Tanaka, M.; Onodera, T.; Wang, J.; Ueda, H.; Toko, K. Array-Based Rational Design of Short Peptide Probe-Derived from an Anti-TNT Monoclonal Antibody. ACS Comb. Sci. 2017, 19, 625–632. [Google Scholar] [CrossRef]
- Wang, J.; Muto, M.; Yatabe, R.; Tahara, Y.; Onodera, T.; Tanaka, M.; Okochi, M.; Toko, K. Highly Selective Rational Design of Peptide-Based Surface Plasmon Resonance Sensor for Direct Determination of 2,4,6-trinitrotoluene (TNT) Explosive. Sens. Actuators B Chem. 2018, 264, 279–284. [Google Scholar] [CrossRef]
- Wang, J.; Muto, M.; Yatabe, R.; Onodera, T.; Tanaka, M.; Okochi, M.; Toko, K. Rational design of peptide-functionalized surface plasmon resonance sensor for specific detection of TNT explosive. Sensors 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.; Fagan, J.A.; Huh, J.Y.; Migler, K.B.; Karim, A.; Raghavan, D. SPR imaging study of DNA wrapped single wall carbon nanotube (ssDNA-SWCNT) adsorption on a model biological (collagen) substrate. Soft Matter 2010, 6, 5581–5588. [Google Scholar] [CrossRef]
- Kakenov, N.; Balci, O.; Balci, S.; Kocabas, C. Probing molecular interactions on carbon nanotube surfaces using surface plasmon resonance sensors. Appl. Phys. Lett. 2012, 101, 223114. [Google Scholar] [CrossRef] [Green Version]
- Sadrolhosseini, A.R.; Noor, A.S.M.; Bahrami, A.; Lim, H.N.; Talib, Z.A.; Mahdi, M.A. Application of polypyrrole multi-walled carbon nanotube composite layer for detection of mercury, lead and iron ions using surface plasmon resonance technique. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Arugula, M.A.; Kirsch, J.S.; Yang, X.; Olsen, E.; Simonian, A.L. Layer-by-layer assembled carbon nanotube-acetylcholinesterase/biopolymer renewable interfaces: SPR and electrochemical characterization. Langmuir 2015, 31, 1462–1468. [Google Scholar] [CrossRef]
- Zorbas, V.; Smith, A.L.; Xie, H.; Ortiz-Acevedo, A.; Dalton, A.B.; Dieckmann, G.R.; Draper, R.K.; Baughman, R.H.; Musselman, I.H. Importance of aromatic content for peptide/single-walled carbon nanotube interactions. J. Am. Chem. Soc. 2005, 127, 12323–12328. [Google Scholar] [CrossRef]
- Johnson, R.R.; Johnson, A.T.C.; Klein, M.L. Probing the structure of DNA-carbon nanotube hybrids with molecular dynamics. Nano Lett. 2008, 8, 69–75. [Google Scholar] [CrossRef]
- Gooding, J.J.; Wibowo, R.; Liu, J.; Yang, W.; Losic, D.; Orbons, S.; Mearns, F.J.; Shapter, J.G.; Hibbert, D.B. Protein electrochemistry using aligned carbon nanotube arrays. J. Am. Chem. Soc. 2003, 125, 9006–9007. [Google Scholar] [CrossRef]
- Drouvalakis, K.A.; Bangsaruntip, S.; Hueber, W.; Kozar, L.G.; Utz, P.J.; Dai, H. Peptide-coated nanotube-based biosensor for the detection of disease-specific autoantibodies in human serum. Biosens. Bioelectron. 2008, 23, 1413–1421. [Google Scholar] [CrossRef] [Green Version]
- Jaworski, J.W.; Raorane, D.; Huh, J.H.; Majumdar, A.; Lee, S.W. Evolutionary screening of biomimetic coatings for selective detection of explosives. Langmuir 2008, 24, 4938–4943. [Google Scholar] [CrossRef]
- Dower, S.K.; Gettins, P.; Jackson, R.; Dwek, R.A.; Givol, D. The binding of 2,4,6-trinitrophenyl derivatives to the mouse myeloma immunoglobulin A protein MOPC 315. Biochem. J. 1978, 169, 179–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pender, M.J.; Sowards, L.A.; Hartgerink, J.D.; Stone, M.O.; Naik, R.R. Peptide-mediated formation of single-wall carbon nanotube composites. Nano Lett. 2006, 6, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Backes, C.; Bosch, S.; Mundloch, U.; Hauke, F.; Hirsch, A. Density gradient ultracentrifugation on carbon nanotubes according to structural integrity as a foundation for an absolute purity evaluation. ChemPhysChem 2011, 12, 2576–2580. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Jiang, J.; Chen, J.; Zhang, Q.; Lu, Y.; Yao, Y.; Li, S.; Logan Liu, G.; Liu, Q. Smartphone-based portable biosensing system using impedance measurement with printed electrodes for 2,4,6-trinitrotoluene (TNT) detection. Biosens. Bioelectron. 2015, 70, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Nergiz, S.Z.; Gandra, N.; Farrell, M.E.; Tian, L.; Pellegrino, P.M.; Singamaneni, S. Biomimetic SERS substrate: Peptide recognition elements for highly selective chemical detection in chemically complex media. J. Mater. Chem. A 2013, 1, 6543–6549. [Google Scholar] [CrossRef]
- Cerruti, M.; Jaworski, J.; Raorane, D.; Zueger, C.; Varadarajan, J.; Carraro, C.; Lee, S.W.; Maboudian, R.; Majumdar, A. Polymer-oligopeptide composite coating for selective detection of explosives in water. Anal. Chem. 2009, 81, 4192–4199. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, A.; Havivi, E.; Shacham, R.; Hahamy, E.; Leibovich, R.; Pevzner, A.; Krivitsky, V.; Davivi, G.; Presman, I.; Elnathan, R.; et al. Supersensitive fingerprinting of explosives by chemically modified nanosensors arrays. Nat. Commun. 2014, 5, 4195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, A.; Zhu, W.; Shang, J.; Klootwijk, J.H.; Sudhölter, E.J.R.; Huskens, J.; de Smet, L.C.P.M. Metal-Organic Polyhedra-Coated Si Nanowires for the Sensitive Detection of Trace Explosives. Nano Lett. 2016. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Lee, B.Y.; Jaworski, J.; Yokoyama, K.; Chung, W.J.; Wang, E.; Hong, S.; Majumdar, A.; Lee, S.W. Selective and sensitive TNT sensors using biomimetic polydiacetylene-coated CNT-FETs. ACS Nano 2011, 5, 2824–2830. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, D.; Cottineau, T.; Piazzon, N.; Josset, S.; Schnell, F.; Pronkin, S.N.; Savinova, E.R.; Keller, V. Bio-inspired nanostructured sensor for the detection of ultralow concentrations of explosives. Angew. Chem. Int. Ed. 2012, 51, 5334–5338. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Du, S.; Onodera, T.; Yatabe, R.; Tanaka, M.; Okochi, M.; Toko, K. An SPR Sensor Chip Based on Peptide-Modified Single-Walled Carbon Nanotubes with Enhanced Sensitivity and Selectivity in the Detection of 2,4,6-Trinitrotoluene Explosives. Sensors 2018, 18, 4461. https://doi.org/10.3390/s18124461
Wang J, Du S, Onodera T, Yatabe R, Tanaka M, Okochi M, Toko K. An SPR Sensor Chip Based on Peptide-Modified Single-Walled Carbon Nanotubes with Enhanced Sensitivity and Selectivity in the Detection of 2,4,6-Trinitrotoluene Explosives. Sensors. 2018; 18(12):4461. https://doi.org/10.3390/s18124461
Chicago/Turabian StyleWang, Jin, Sanyang Du, Takeshi Onodera, Rui Yatabe, Masayoshi Tanaka, Mina Okochi, and Kiyoshi Toko. 2018. "An SPR Sensor Chip Based on Peptide-Modified Single-Walled Carbon Nanotubes with Enhanced Sensitivity and Selectivity in the Detection of 2,4,6-Trinitrotoluene Explosives" Sensors 18, no. 12: 4461. https://doi.org/10.3390/s18124461





