Dual-Mode Gas Sensor Composed of a Silicon Nanoribbon Field Effect Transistor and a Bulk Acoustic Wave Resonator: A Case Study in Freons
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
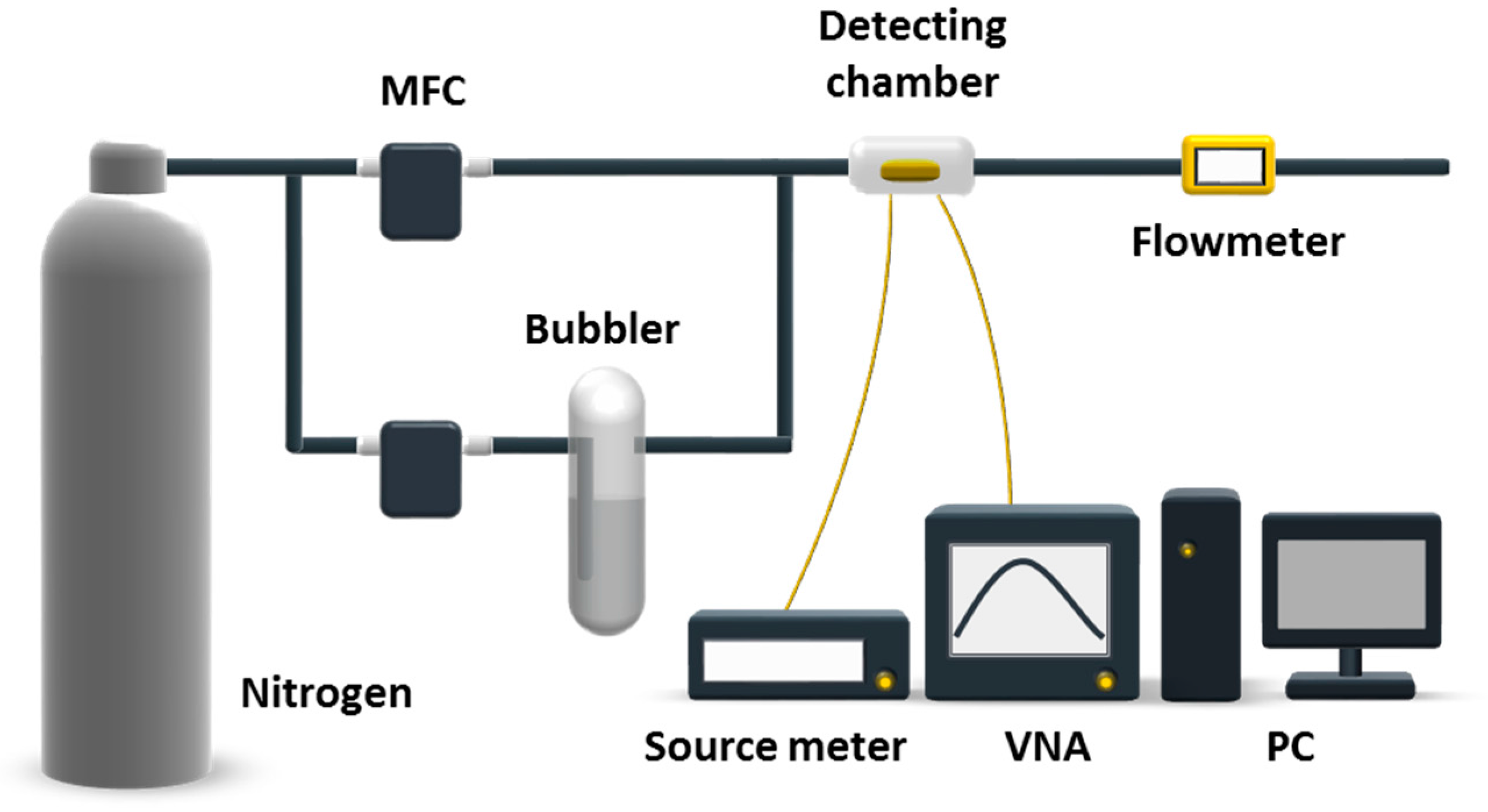
2.2. Experimental Setup
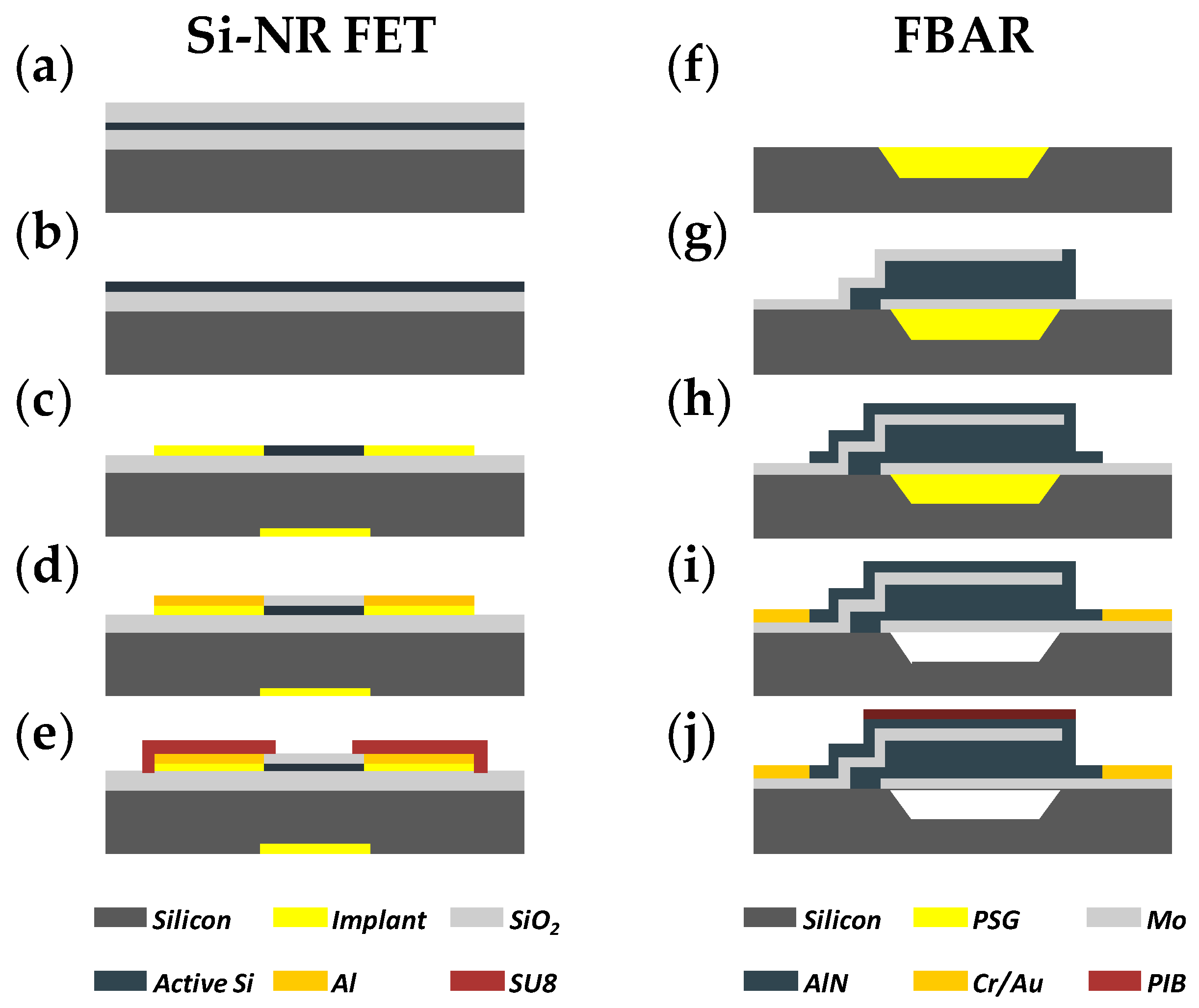
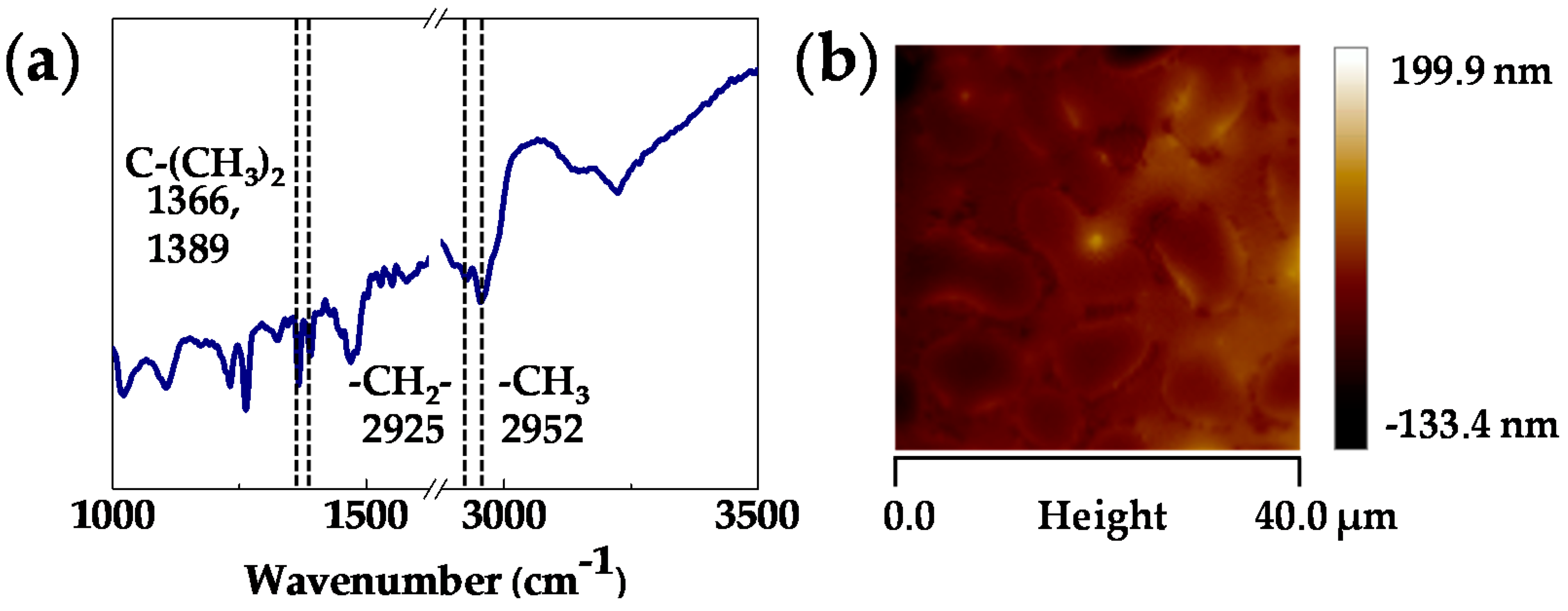
2.3. Device Fabrication and Functionalization
3. Results
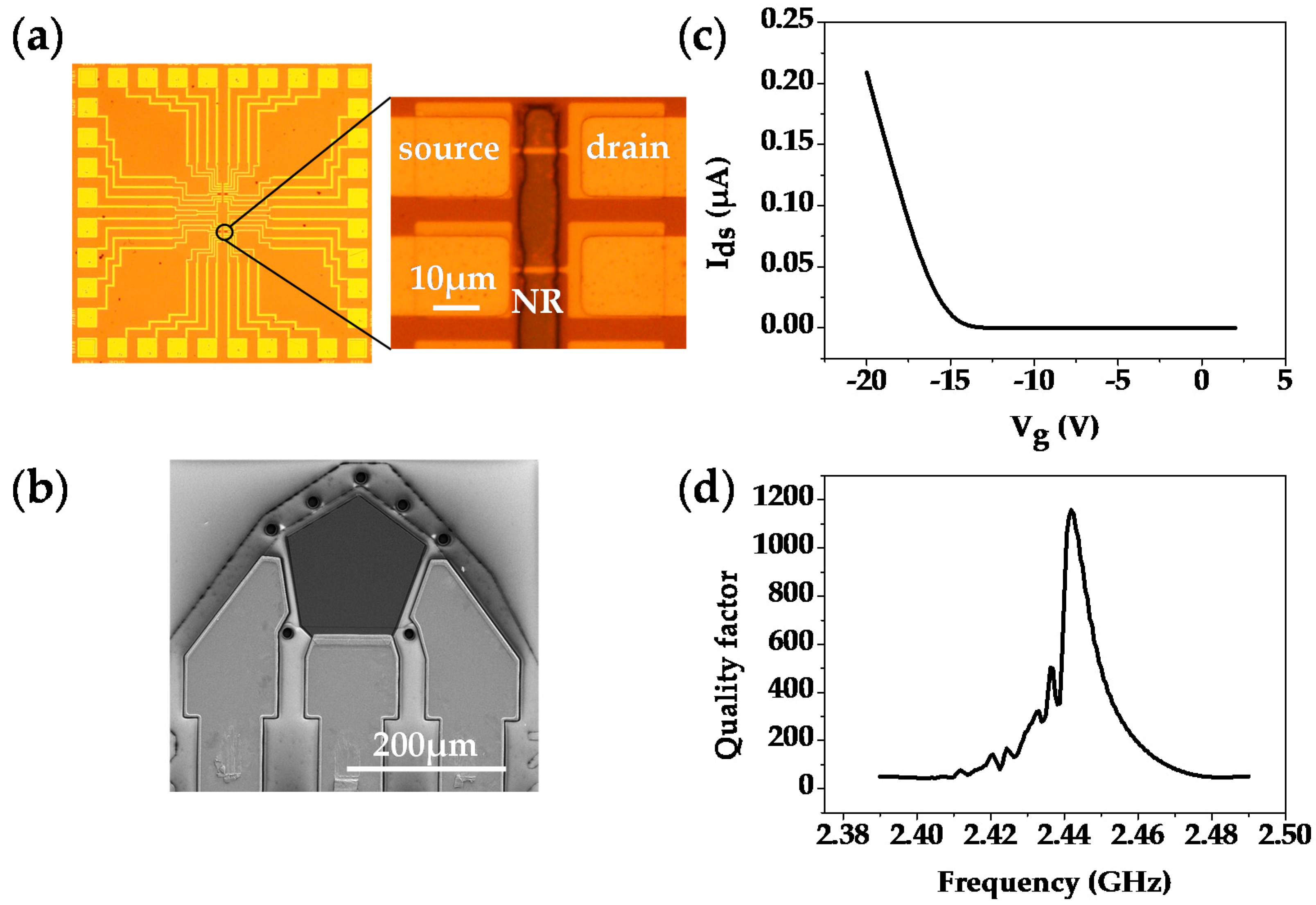
3.1. Sensor Characterizations
3.2. Si-NR FET Results
3.3. FBAR Results
3.4. Mixture Analysis with the Dual-Mode Gas Sensor
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Arshak, K.; Moore, E.; Lyons, G.M.; Harris, J.; Clifford, S. A review of gas sensors employed in electronic nose applications. Sens. Rev. 2004, 24, 181–198. [Google Scholar] [CrossRef]
- Muñoz, R.; Sivret, E.C.; Parcsi, G.; Lebrero, R.; Wang, X.; Suffet, I.H.; Stuetz, R.M. Monitoring techniques for odour abatement assessment. Water Res. 2010, 44, 5129–5149. [Google Scholar] [CrossRef] [PubMed]
- Fanget, S.; Hentz, S.; Puget, P.; Arcamone, J.; Matheron, M.; Colinet, E.; Andreucci, P.; Duraffourg, L.; Myers, E.; Roukes, M.L. Gas sensors based on gravimetric detection—A review. Sens. Actuators B Chem. 2011, 160, 804–821. [Google Scholar] [CrossRef]
- Stetter, J.R.; Penrose, W.R.; Yao, S. Sensors, chemical sensors, electrochemical sensors, and ecs. J. Electrochem. Soc. 2003, 150, S11–S16. [Google Scholar] [CrossRef]
- Gebicki, J. Application of electrochemical sensors and sensor matrixes for measurement of odorous chemical compounds. TrAC Trends Anal. Chem. 2016, 77, 1–13. [Google Scholar] [CrossRef]
- Rakow, N.A.; Suslick, K.S. A colorimetric sensor array for odour visualization. Nature 2000, 406, 710–713. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.; Porto-Figueira, P.; Cavaco, C.; Taunk, K.; Rapole, S.; Dhakne, R.; Nagarajaram, H.; Câmara, J.S. Breath analysis as a potential and non-invasive frontier in disease diagnosis: An overview. Metabolites 2015, 5, 3–55. [Google Scholar] [CrossRef] [PubMed]
- Guentner, A.T.; Koren, V.; Chikkadi, K.; Righettoni, M.; Pratsinis, S.E. E-nose sensing of low-ppb formaldehyde in gas mixtures at high relative humidity for breath screening of lung cancer? ACS Sens. 2016, 1, 528–535. [Google Scholar] [CrossRef]
- Cynkar, W.; Dambergs, R.; Smith, P.; Cozzolino, D. Classification of tempranillo wines according to geographic origin: Combination of mass spectrometry based electronic nose and chemometrics. Anal. Chim. Acta 2010, 660, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, S.; Panigrahi, S.; Mallik, S. Olfactory receptor based piezoelectric biosensors for detection of alcohols related to food safety applications. Sens. Actuators B Chem. 2011, 155, 8–18. [Google Scholar] [CrossRef]
- Compagnone, D.; Fusella, G.C.; Carlo, D.M.; Pittia, P.; Martinelli, E.; Tortora, L.; Paolesse, R.; Natale, D.C. Gold nanoparticles-peptide based gas sensor arrays for the detection of foodaromas. Biosens. Bioelectron. 2013, 42, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.; Petersen, M.A.; Byrne, D.V. Sensory based quality control utilising an electronic nose and GC-MS analyses to predict end-product quality from raw materials. Meat Sci. 2005, 69, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Varghese, O.K.; Gong, D.; Dreschel, W.R.; Ong, K.G.; Grimes, C.A. Ammonia detection using nanoporous alumina resistive and surface acoustic wave sensors. Sens. Actuators B Chem. 2003, 94, 27–35. [Google Scholar] [CrossRef]
- Bachar, N.; Liberman, L.; Muallem, F.; Feng, X.; Mullen, K.; Haick, H. Sensor arrays based on polycyclic aromatic hydrocarbons: Chemiresistors versus quartz-crystal microbalance. ACS Appl. Mater. Interfaces 2013, 5, 11641–11653. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, H.; Feng, Z.; Zhang, H.; Zhang, R.; Yu, Y.; Tao, J.; Zhao, H.; Guo, W.; Pang, W.; et al. Chemiresistive and gravimetric dual-mode gas sensor toward target recognition and differentiation. ACS Appl. Mater. Interfaces 2016, 8, 21742–21749. [Google Scholar] [CrossRef] [PubMed]
- Paska, Y.; Stelzner, T.; Christiansen, S.; Haick, H. Enhanced sensing of nonpolar volatile organic compounds by silicon nanowire field effect transistors. ACS Nano 2011, 5, 5620–5626. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Haick, H. Effect of functional groups on the sensing properties of silicon nanowires toward volatile compounds. ACS Appl. Mater. Interfaces 2013, 5, 2289–2299. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sun, Z.; Lu, Q.; Zeng, F.; Su, D. One-Pot Synthesis of (Au Nanorod)-(Metal Sulfide) Core-Shell Nanostructures with Enhanced Gas-Sensing Property. Small 2012, 8, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Majhi, S.; Rai, P.; Raj, S.; Chon, B.-S.; Park, K.-K.; Yu, Y.-T. Effect of Au Nanorods on Potential Barrier Modulation in Morphologically Controlled Au@Cu2O Core-Shell Nanoreactors for Gas Sensor Applications. ACS Appl. Mater. Interfaces 2014, 6, 7491–7497. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.J.; Franklin, N.R.; Kong, J.; Cao, J.; Tombler, T.W.; Zhang, Y.; Dai, H. Molecular photodesorption from single-walled carbon nanotubes. Appl. Phys. Lett. 2001, 79, 2258–2260. [Google Scholar] [CrossRef]
- Nakhleh, M.K.; Amal, H.; Jeries, R.; Broza, Y.Y.; Aboud, M.; Gharra, A.; Ivgi, H.; Khatib, S.; Badarneh, S.; Har-Shai, L.; et al. Diagnosis and classification of 17 diseases from 1404 subjects via pattern analysis of exhaled molecules. ACS Nano 2017, 11, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Hong, M.; Wang, L.; Huang, K.; Guo, Y. Synthesis and gas-sensing properties of P-type Na0.44MnO2 nanoribbons. Mater. Lett. 2016, 164, 440–443. [Google Scholar] [CrossRef]
- Clavaguera, S.; Carella, A.; Caillier, L.; Celle, C.; Pécaut, J.; Lenfant, S.; Vuillaume, D.; Simonato, J.P. Sub-ppm detection of nerve agents using chemically functionalized silicon nanoribbon field-effect transistors. Angew. Chem. Int. Ed. 2010, 49, 4063–4066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ippolito, S.J.; Trinchi, A.; Powell, D.A.; Wlodarski, W. Acoustic wave gas and vapor sensors. In Solid State Gas Sensing; Springer: Berlin, Germany, 2009; pp. 1–44. [Google Scholar]
- Benetti, M.; Cannatà, D.; Pietrantonio, D.F.; Foglietti, V.; Verona, E. Microbalance chemical sensor based on thin-film bulk acoustic wave resonators. Appl. Phys. Lett. 2005, 87, 173504. [Google Scholar] [CrossRef]
- Liu, W.; Wang, J.; Yu, Y.; Chang, Y.; Tang, N.; Qu, H.; Wang, Y.; Pang, W.; Zhang, H.; Zhang, D.; et al. Tuning the resonant frequency of resonators using molecular surface self-assembly approach. ACS Appl. Mater. Interfaces 2015, 7, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chang, Y.; Tang, N.; Qu, H.; Liu, J.; Pang, W.; Zhang, H.; Zhang, D.; Duan, X. Detection of volatile organic compounds using microfabricated resonator array functionalized with supramolecular monolayers. ACS Appl. Mater. Interfaces 2015, 7, 17893–17903. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Tang, N.; Qu, H.; Liu, J.; Zhang, D.; Zhang, H.; Pang, W.; Duan, X. Detection of volatile organic compounds by self-assembled monolayer coated sensor array with concentration-independent fingerprints. Sci. Rep. 2016, 6, 23970. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Qu, H.; Hu, J.; Pang, W.; Zhang, H.; Duan, X. A highly sensitive humidity sensor based on ultrahigh-frequency microelectromechanical resonator coated with nano-assembled polyelectrolyte thin films. Micromachines 2017, 8, 116. [Google Scholar] [CrossRef]
- Lin, R.-C.; Chen, Y.-C.; Chang, W.-T.; Cheng, C.-C.; Kao, K.-S. Highly sensitive mass sensor using film bulk acoustic resonator. Sens. Actuators A Phys. 2008, 147, 425–429. [Google Scholar] [CrossRef]
- Nirschl, M.; Schreiter, M.; Vörös, J. Comparison of fbar and qcm-d sensitivity dependence on adlayer thickness and viscosity. Sens. Actuators A Phys. 2011, 165, 415–421. [Google Scholar] [CrossRef] [Green Version]
- Wallington, T.J.; Schneider, W.F.; Worsnop, D.R.; Nielsen, O.J.; Sehested, J.; Debruyn, W.J.; Shorter, J.A. The environmental impact of CFC replacements—HFCs and HCFCs. Environ. Sci. Technol. 1994, 28, 320A–326A. [Google Scholar] [PubMed]
- Tsai, W.-T. A review of environmental hazards and adsorption recovery of cleaning solvent hydrochlorofluorocarbons (HCFCs). J. Loss Prev. Process Indust. 2002, 15, 147–157. [Google Scholar] [CrossRef]
- Mangani, F.; Maione, M.; Lattanzi, L.; Arduini, J. A gas chromatographic-mass spectrometric method for trace analysis of chlorofluorocarbons and their replacement compounds in atmospheric samples. Chromatographia 2000, 51, 325–330. [Google Scholar] [CrossRef]
- Mangani, F.; Maione, M.; Lattanzi, L.; Arduini, J. Atmospheric measurements of the halogenated hydrocarbons involved in global change phenomena. Atmos. Environ. 2000, 34, 5303–5309. [Google Scholar] [CrossRef]
- Brown, A.T.; Chipperfield, M.P.; Boone, C.; Wilson, C.; Walker, K.A.; Bernath, P.F. Trends in atmospheric halogen containing gases since 2004. J. Quant. Spectrosc. Radiat. Transf. 2011, 112, 2552–2566. [Google Scholar] [CrossRef]
- Bris, K.; McDowell, J.; Strong, K. Measurements of the infrared absorption cross-sections of HCFC-141b (CH3CFCL2). J. Quant. Spectrosc. Radiat. Transf. 2012, 113, 1913–1919. [Google Scholar] [CrossRef]
- Belton, P.S.; Saffa, A.M.; Wilson, R.H.; Ince, A.D. The potential of fourier transform infrared spectroscopy for the analysis of confectionery products. Food Chem. 1988, 28, 53–61. [Google Scholar] [CrossRef]
- Castillo, S.; Mattila, I.; Miettinen, J.; Orešič, M.; Hyötyläinen, T. Data analysis tool for comprehensive two-dimensional gas chromatography/time-of-flight mass spectrometry. Anal. Chem. 2011, 83, 3058–3067. [Google Scholar] [CrossRef] [PubMed]
- Ul-Haq, Z.; Ali, M.; Batool, S.; Tariq, S.; Qayyum, Z. Emission quantification of refrigerant CFCs, HCFCs and HFCs in megacity Lahore (Pakistan) and contributed ODPs and GWPs. J. Earth Syst. Sci. 2016, 125, 1273–1284. [Google Scholar] [CrossRef]
- Abbasi, S.A.; Abbasi, T.; Abbasi, S.A.; Abbasi, T. Ozone Hole; Springer-Verlag NY Inc.: New York, NY, USA, 2017; pp. 37–50. [Google Scholar]
- Nguyen, H.; El-Safty, S.A. Meso- and macroporous Co3O4 nanorods for effective voc gas sensors. J. Phys. Chem. C 2011, 115, 8466–8474. [Google Scholar] [CrossRef]
- Duan, X.; Li, Y.; Rajan, N.K.; Routenberg, D.A.; Modis, Y.; Reed, M.A. Quantification of the affinities and kinetics of protein interactions using silicon nanowire biosensors. Nat. Nanotechnol. 2012, 7, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Sauerbrey, G. Use of vibrating quartz for thin film weighing and microweighing. Z. Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Pang, W.; Zhao, H.; Kim, E.; Zhang, H.; Yu, H.; Hu, X. Piezoelectric microelectromechanical resonant sensors for chemical and biological detection. Lab Chip 2011, 12, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, S.; Ng, E.; Ahn, C.; Yang, Y.; Wang, S.; Hong, V.A.; Kenny, T.W. Accurate modeling of quality factor behavior of complex silicon mems resonators. J. Microelectromech. Syst. 2015, 24, 276–288. [Google Scholar] [CrossRef]
- Hwang, M.-J.; Shim, W.; Ryu, D.-W.; Moon, H. Low-pressure adsorption isotherms of aromatic compounds on polyisobutylene gel measured on a quartz crystal microbalance. J. Chem. Eng. Data 2012, 57, 701–707. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Zhu, C.-L.; Xiao, G. Ethanol sensing characteristics of ambient temperature sonochemically synthesized zno nanotubes. Sens. Actuators B Chem. 2008, 129, 639–642. [Google Scholar] [CrossRef]
- Schmidt, M.W.; Baldridge, K.K.; Boatz, J.A.; Elbert, S.T.; Gordon, M.S.; Jensen, J.H.; Koseki, S.; Matsunaga, N.; Nguyen, K.A.; Su, S. General atomic and molecular electronic structure system. J. Comput. Chem. 1993, 14, 1347–1363. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Seri-Levy, A.; Avnir, D. The brunauer-emmett-teller equation and the effects of lateral interactions. A simulation study. Langmuir 1993, 9, 2523–2529. [Google Scholar] [CrossRef]
- Iqbal, N.; Mustafa, G.; Rehman, A.; Biedermann, A.; Najafi, B.; Lieberzeit, P.A.; Dickert, F.L. QCM-arrays for sensing terpenes in fresh and dried herbs via bio-mimetic mip layers. Sensors 2010, 10, 6361–6376. [Google Scholar] [CrossRef] [PubMed]

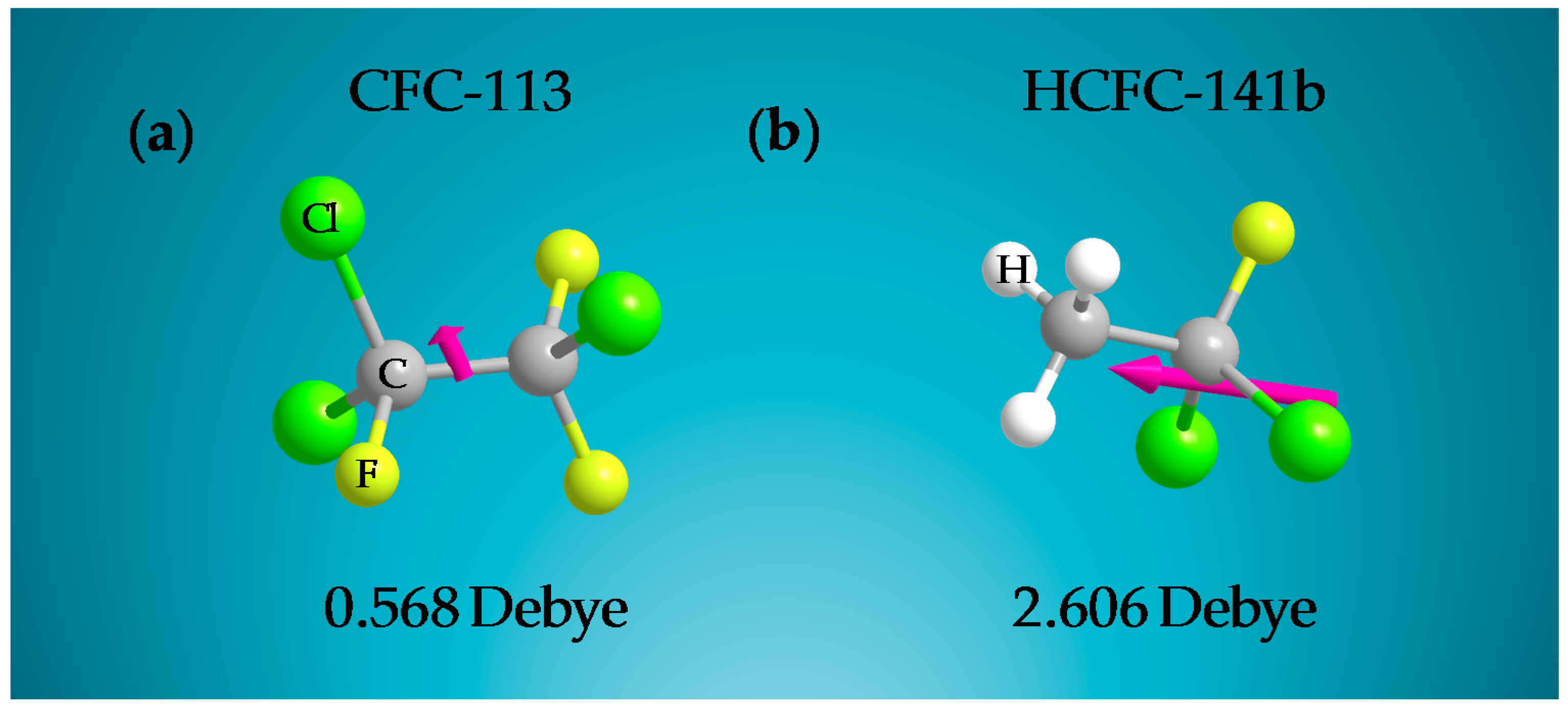
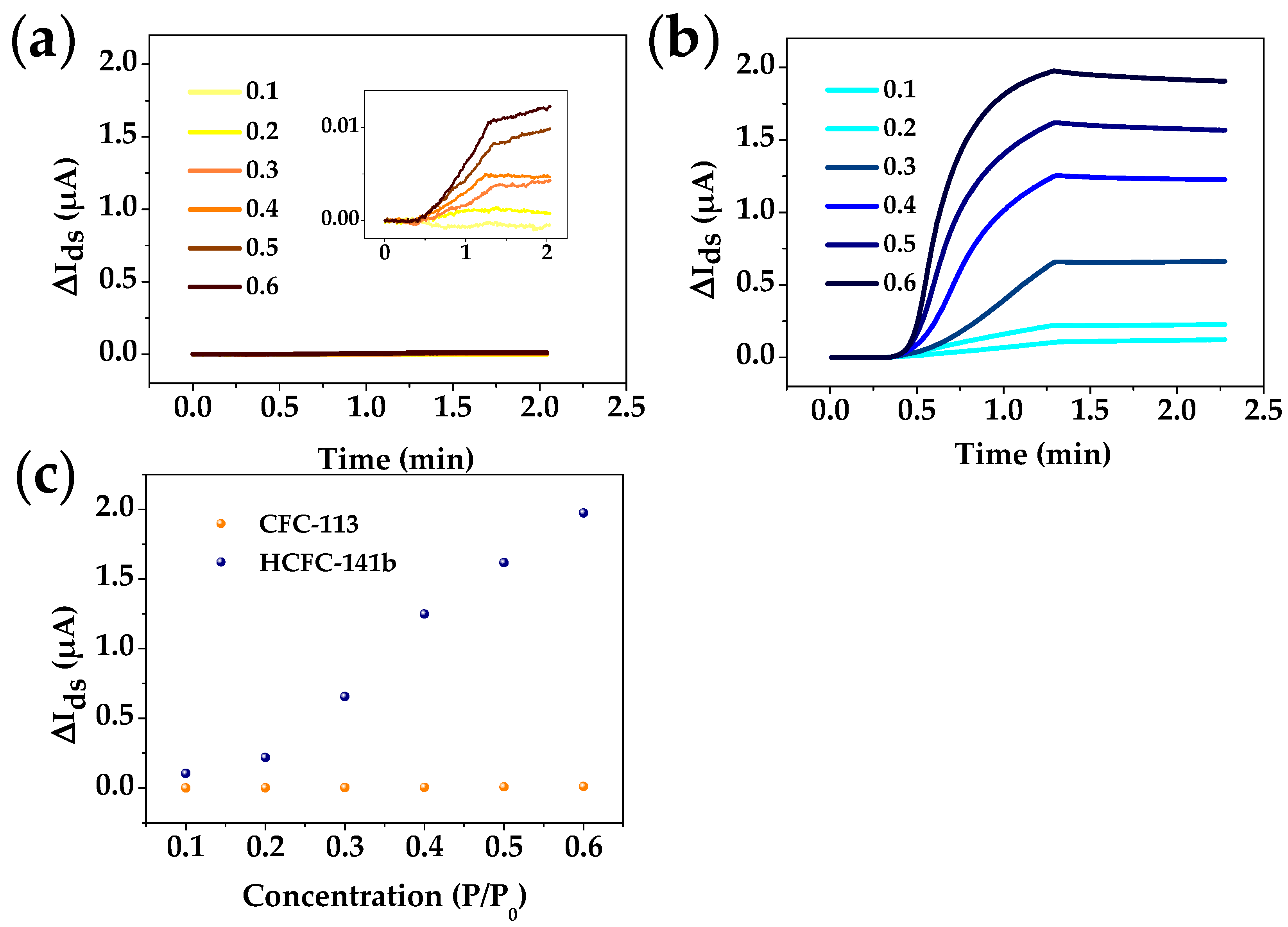
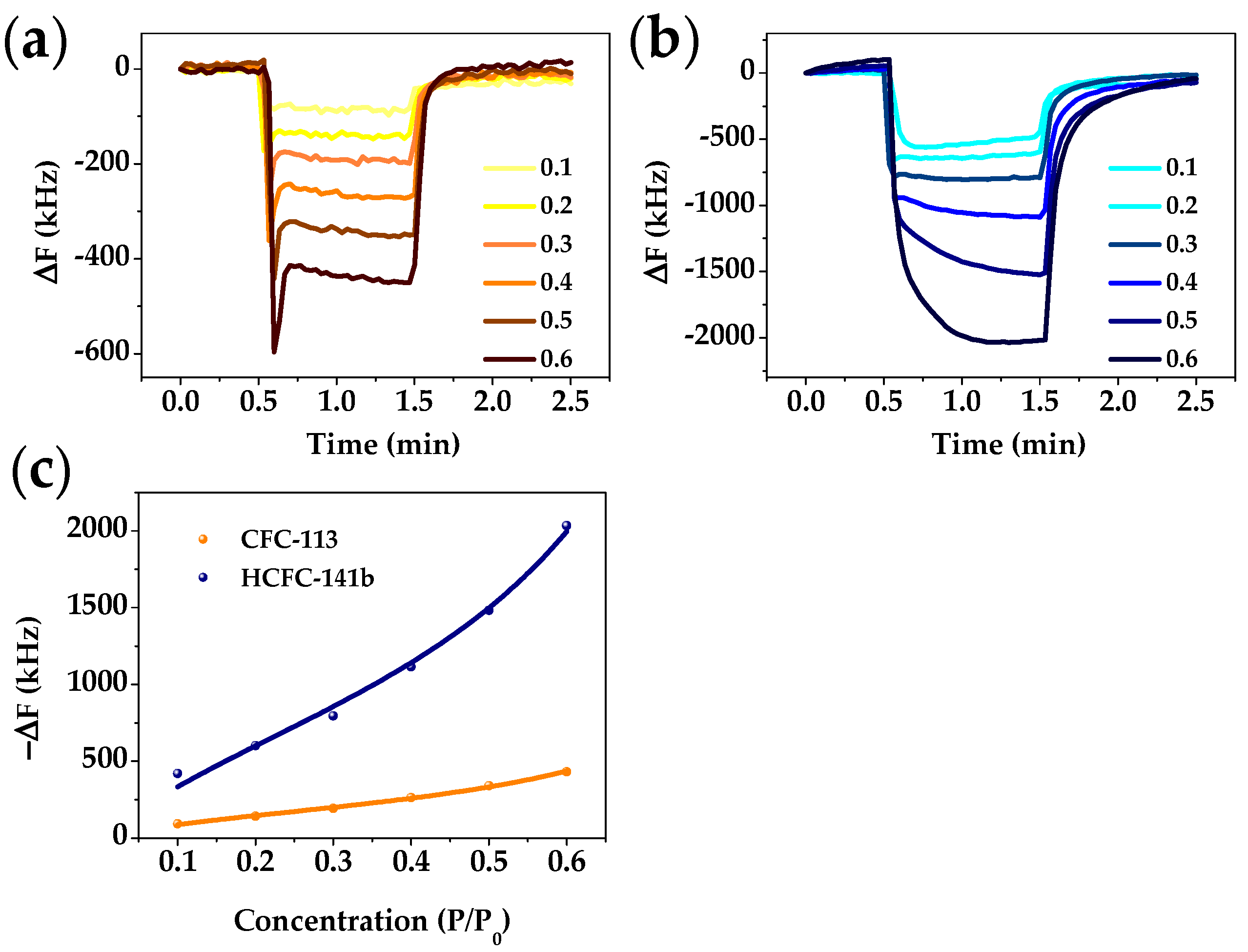
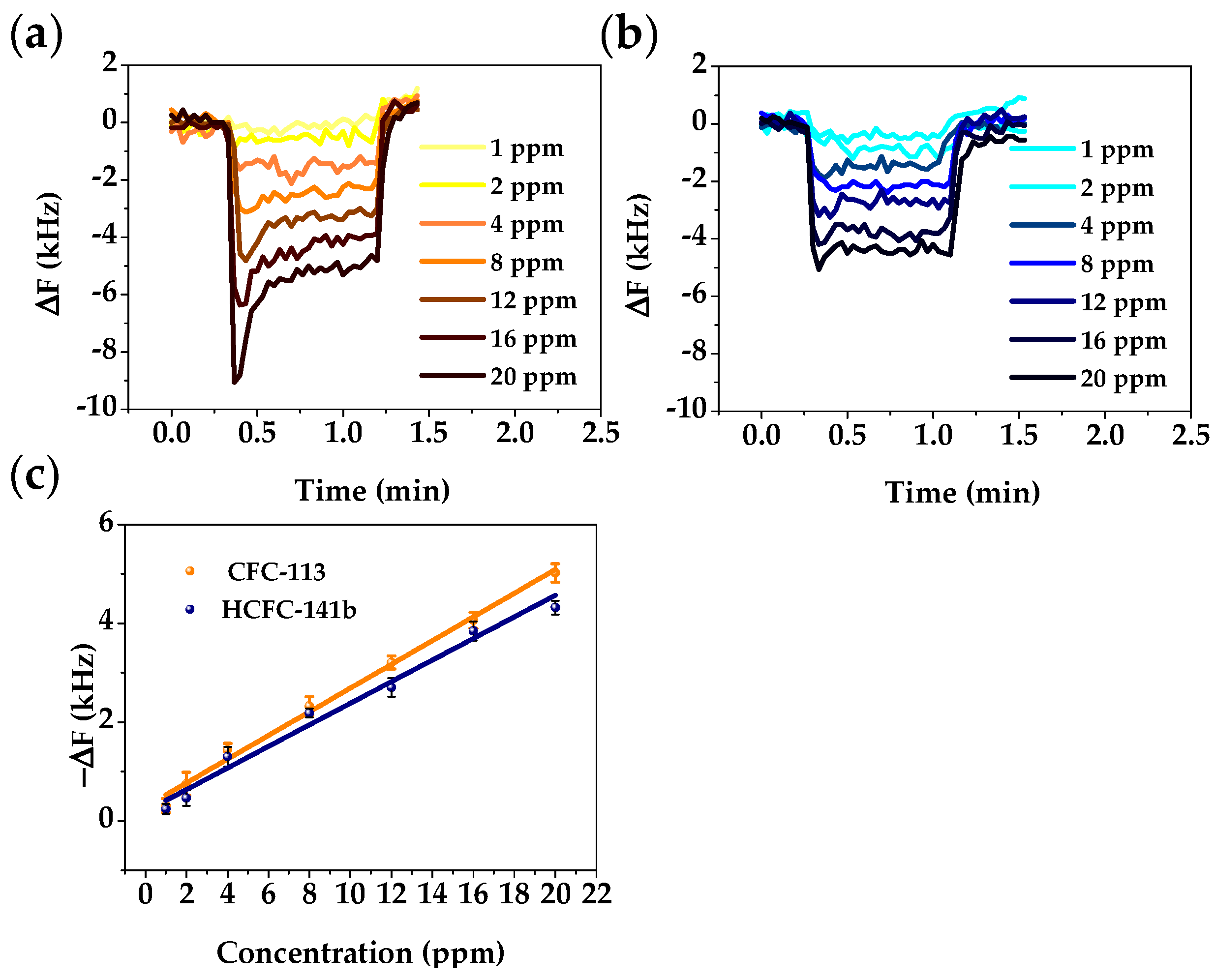

| Freon | Atmospheric Lifetime (Years) | ODP | GWP | Volume 1 (Å3) | Boiling Point (K) | Critical Pressure (MPa) |
|---|---|---|---|---|---|---|
| CFC-113 | 93 | 0.81 | 6080 | 96.142 | 320.754 | 3.820 |
| HCFC-141b | 9.4 | 0.11 | 717 | 74.304 | 300.129 | 4.386 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.; Hui, Z.; Wang, X.; Qu, H.; Pang, W.; Duan, X. Dual-Mode Gas Sensor Composed of a Silicon Nanoribbon Field Effect Transistor and a Bulk Acoustic Wave Resonator: A Case Study in Freons. Sensors 2018, 18, 343. https://doi.org/10.3390/s18020343
Chang Y, Hui Z, Wang X, Qu H, Pang W, Duan X. Dual-Mode Gas Sensor Composed of a Silicon Nanoribbon Field Effect Transistor and a Bulk Acoustic Wave Resonator: A Case Study in Freons. Sensors. 2018; 18(2):343. https://doi.org/10.3390/s18020343
Chicago/Turabian StyleChang, Ye, Zhipeng Hui, Xiayu Wang, Hemi Qu, Wei Pang, and Xuexin Duan. 2018. "Dual-Mode Gas Sensor Composed of a Silicon Nanoribbon Field Effect Transistor and a Bulk Acoustic Wave Resonator: A Case Study in Freons" Sensors 18, no. 2: 343. https://doi.org/10.3390/s18020343





