Three-Dimensional Blood Vessel Model with Temperature-Indicating Function for Evaluation of Thermal Damage during Surgery
Abstract
:1. Introduction
2. Concept of Functional Blood Vessel Model
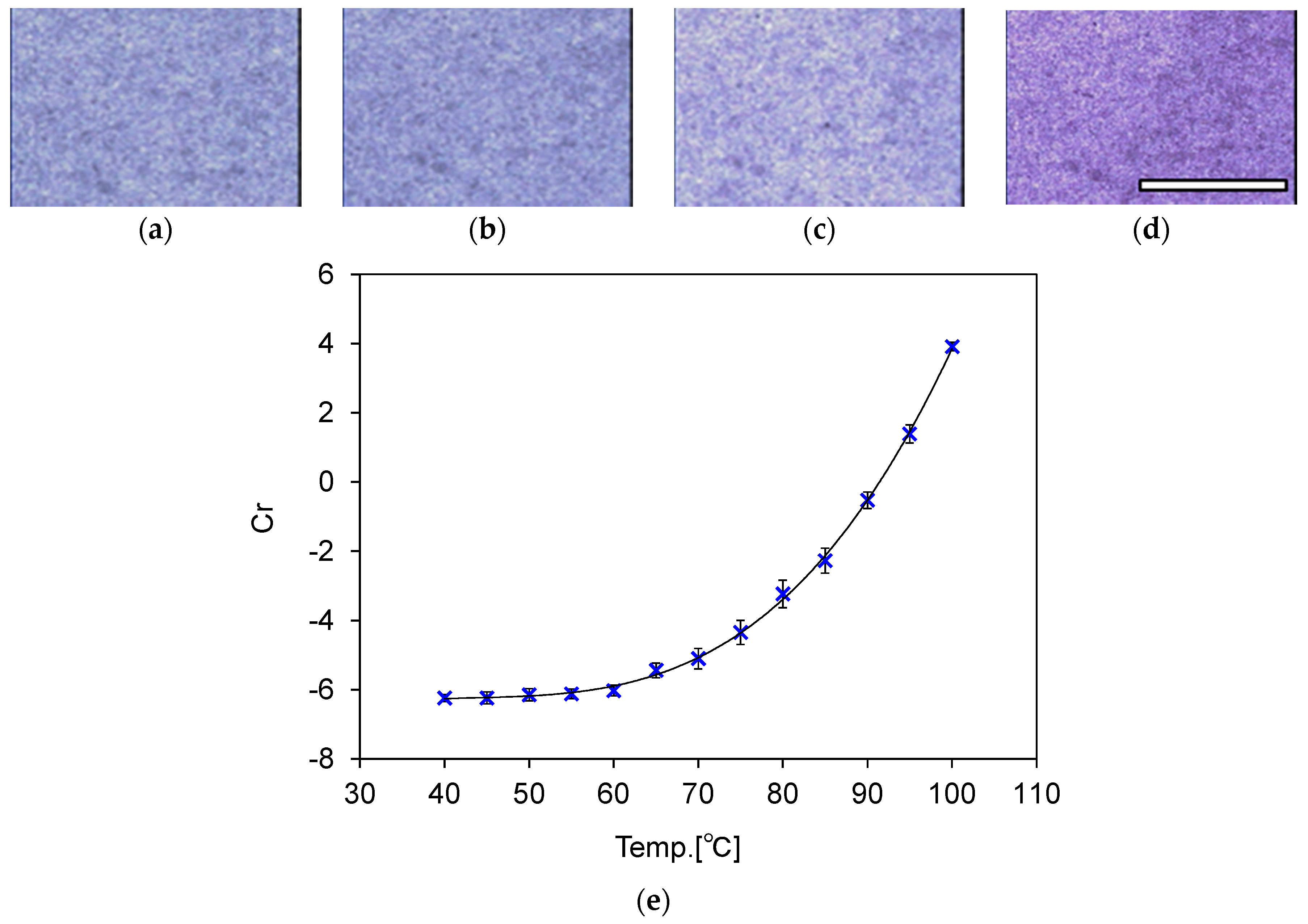
3. Evaluation of Temperature-Indicating Function
3.1. Preparation of Composite Material
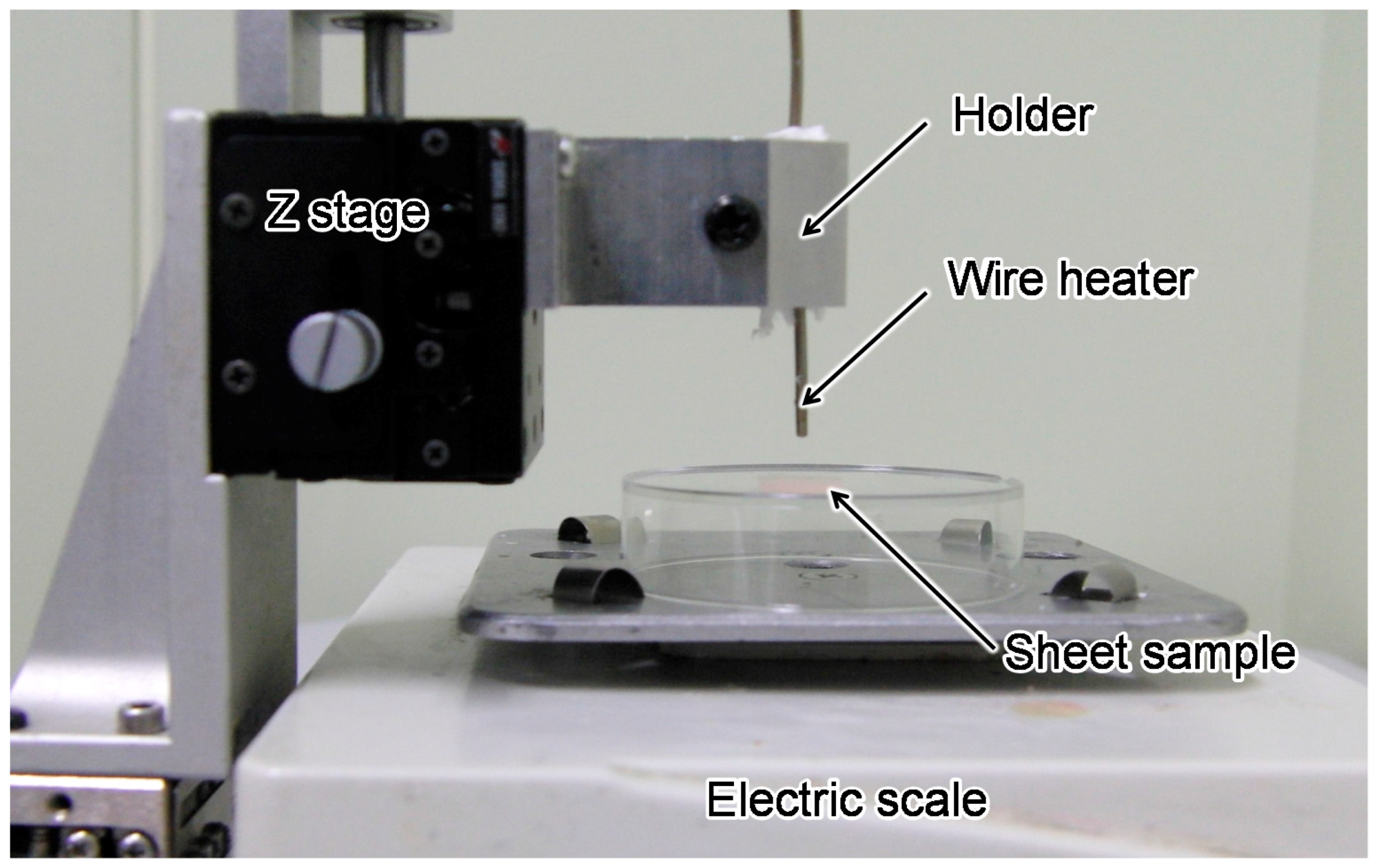
3.2. Measurement System Setup
3.3. Calibration of Temperature and Color Information
4. 3D Blood Vessel Model with Temperature-Indicating Function
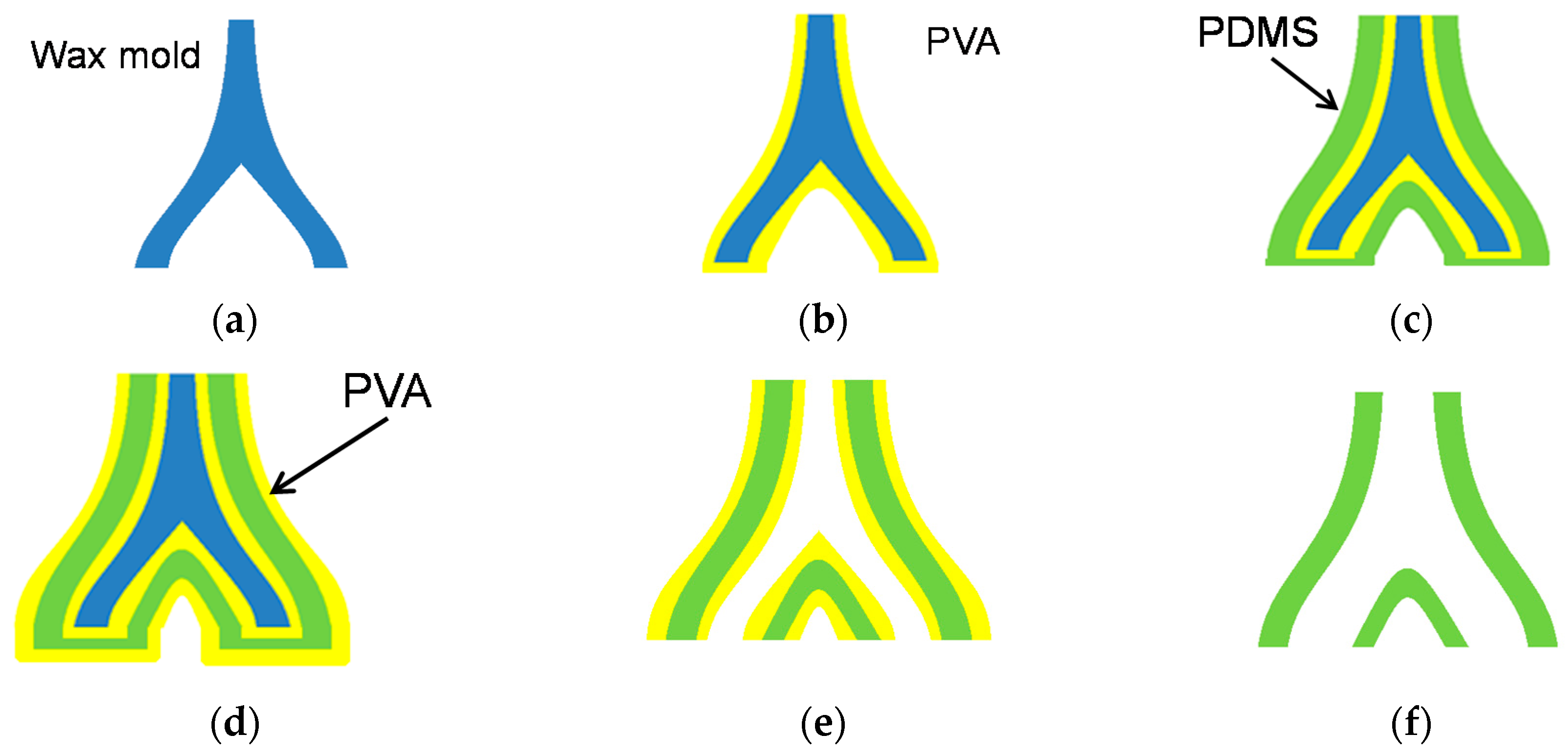
4.1. Fabrication Process
- (a)
- A solid wax mold is made in the shape of a blood vessel using a wax 3D printer (3Z PRO; Solidscape Inc., Merrimack, NH, USA). Structural data of blood vessel were first obtained as 2D tomographic data of DICOM format by using X-ray CT scanning. Then, 3D data of STL format were generated based on these 2D data. The data were provided by Dr. Seiichi Ikeda. For more detailed explanation about this process, please confirm Reference [14].
- (b)
- 8 wt % polyvinyl alcohol (PVA) dissolved in water is dip-coated onto the wax mold as a protective layer. The model is then dried at room temperature for 2 h. This step is repeated four times to acquire a sufficient PVA film thickness. Evaluation of the condition of the PVA thickness is described in detail in the next subsection.
- (c)
- The composite material of PDMS and the thermochromic dye is dip-coated onto the mold and cured at room temperature for 10 h. This step is repeated six times.
- (d)
- 8 wt % PVA is coated onto the model four more times.
- (e)
- The wax mold is removed using acetone.
- (f)
- The coated PVA is removed by water.
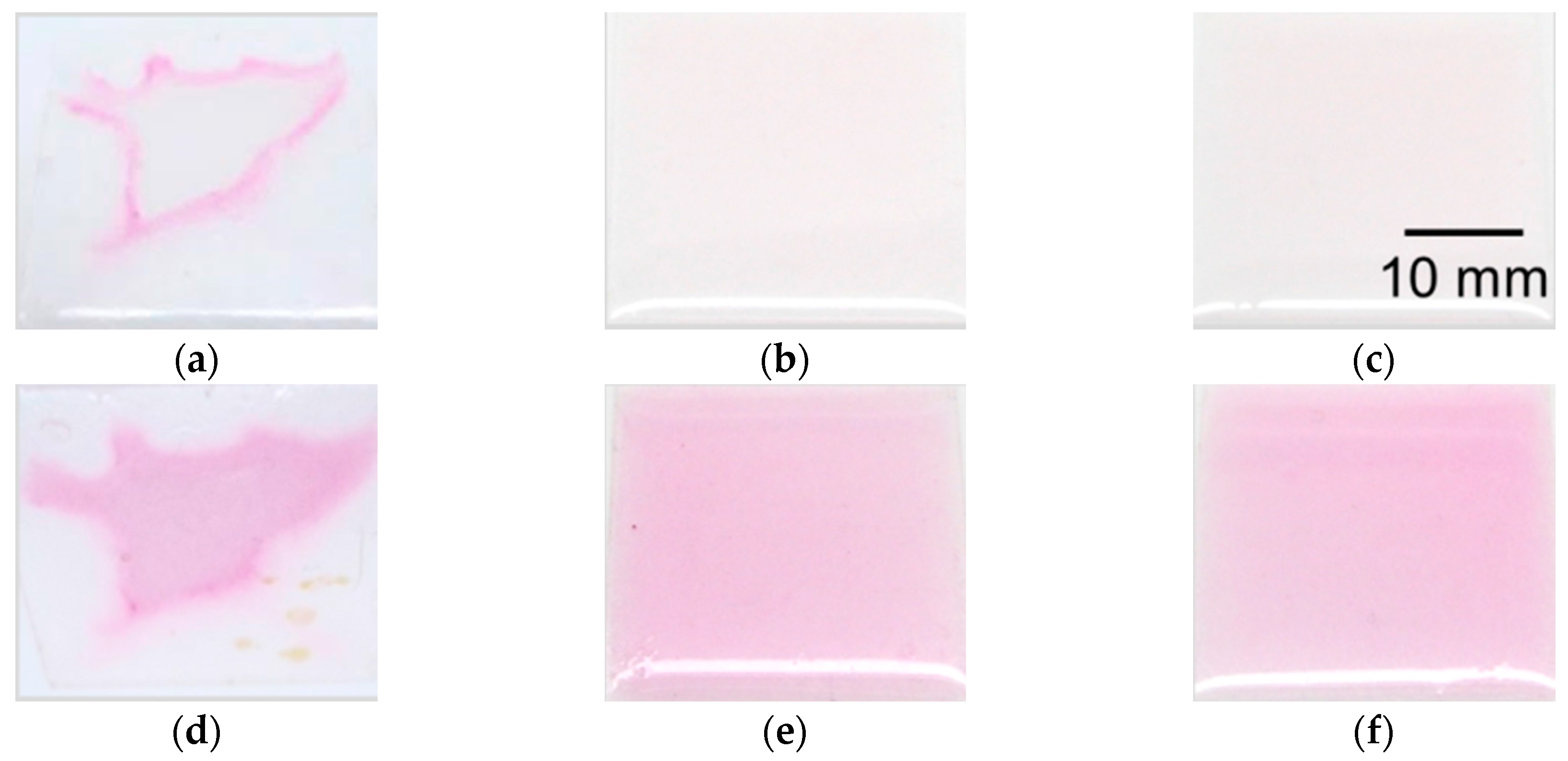
4.2. Evaluation of Coating Condition of PVA
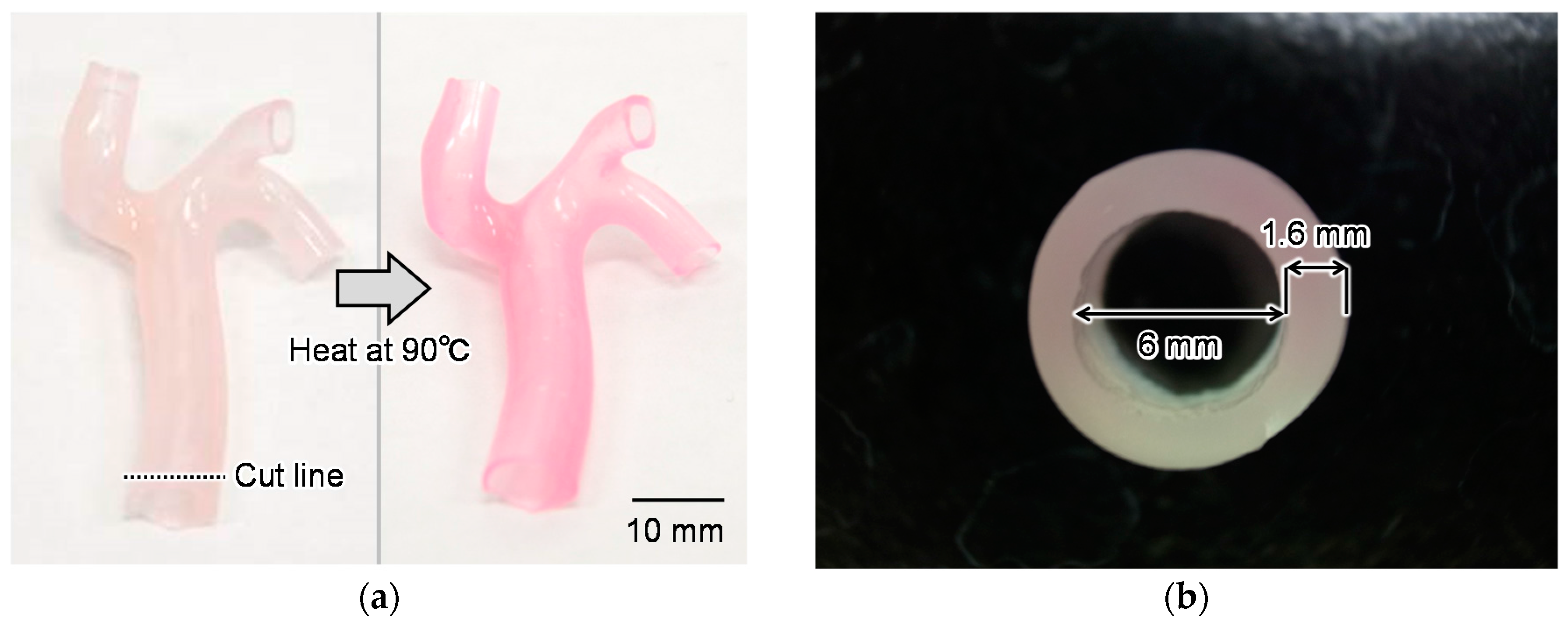
4.3. Fabrication Results
5. Discussion
5.1. Measurement Errors and Spatial Resolution of the Temperature Measurement
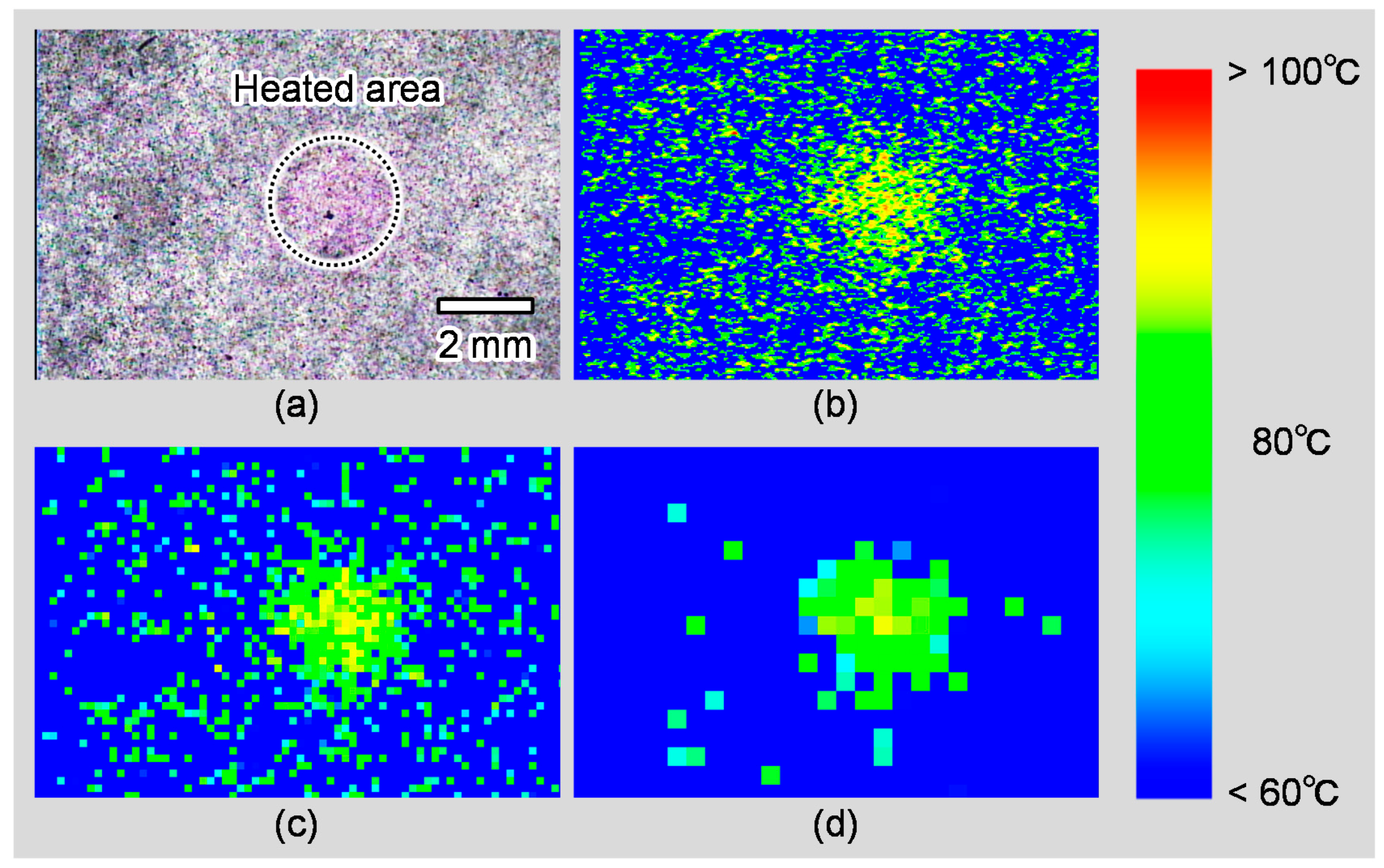
5.2. Evaluation of Local Heating
5.3. Thermal or Electrical Characteristics of the Model
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| PDMS | polydimethylsiloxane |
| PVA | polyvinyl alcohol |
| 3D | three-dimensional |
Appendix A
| Temperature (°C) | Error (°C) |
|---|---|
| 60 | 2.40 |
| 65 | −1.64 |
| 70 | 0.07 |
| 75 | −0.26 |
| 80 | −0.86 |
| 85 | 0.37 |
| 90 | −0.11 |
| 95 | 0.04 |
| 100 | −0.22 |
References
- Saifi, J.; Chang, B.B.; Paty, P.S.; Kaufrnan, J.; Leather, R.P.; Shah, D.M. An animal model for instructing and the study of in situ arterial bypass. J. Vasc. Surg. 1990, 12, 538–540. [Google Scholar] [CrossRef]
- Shinbane, J.S.; Wood, M.A.; Jensen, D.N.; Ellenbogen, K.A.; Fitzpatrick, A.P.; Scheinman, M.M. Tachycardia-induced cardiomyopathy: A review of animal models and clinical studies. J. Am. Coll. Cardiol. 1997, 29, 709–715. [Google Scholar] [CrossRef]
- Patterson, E.J.; Scudamore, C.H.; Owen, D.A.; Nagy, A.G.; Buczkowski, A.K. Radiofrequency ablation of porcine liver in vivo: Effects of blood flow and treatment time on lesion size. Ann. Surg. 1998, 227, 559. [Google Scholar] [CrossRef] [PubMed]
- Dorrance, H.R.; Oien, K.; O’Dwyer, P.J. Effects of laparoscopy on intraperitoneal tumor growth and distant metastases in an animal model. Surgery 1999, 126, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Satava, R.M. Surgical education and surgical simulation. World. J. Surg. 2001, 25, 1484–1489. [Google Scholar] [CrossRef] [PubMed]
- Kneebone, R. Simulation in surgical training: Educational issues and practical implications. Med. Educ. 2003, 37, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Kunkler, K. The role of medical simulation: An overview. Int. J. Med. Robot. Comput. Assist. Surg. 2006, 2, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Satava, R.M. Historical review of surgical simulation—A personal perspective. World J. Surg. 2008, 32, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Satava, R.M. Virtual reality surgical simulator. Surg. Endosc. 1993, 7, 203–205. [Google Scholar] [CrossRef] [PubMed]
- Krummel, T.M. Surgical simulation and virtual reality: The coming revolution. Ann. Surg. 1998, 228, 635. [Google Scholar] [CrossRef] [PubMed]
- Keyser, E.J.; Derossis, A.M.; Antoniuk, M.; Sigman, H.H.; Fried, G.M. A simplified simulator for the training and evaluation of laparoscopic skills. Surg. Endosc. 2000, 14, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Basdogan, C.; Ho, C.H.; Srinivasan, M.A. Virtual environments for medical training: Graphical and haptic simulation of laparoscopic common bile duct exploration. IEEE/ASME Trans. Mechatron. 2001, 6, 269–285. [Google Scholar] [CrossRef]
- Grober, E.D.; Hamstra, S.J.; Wanzel, K.R.; Reznick, R.K.; Matsumoto, E.D.; Sidhu, R.S.; Jarvi, K.A. The educational impact of bench model fidelity on the acquisition of technical skill: The use of clinically relevant outcome measures. Ann. Surg. 2004, 240, 374. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, S.; Arai, F.; Fukuda, T.; Negoro, M.; Irie, K. An in vitro patient-specific biological model of the cerebral artery reproduced with a membranous configuration for simulating endovascular intervention. J. Robot. Mechatron. 2005, 17, 327–334. [Google Scholar] [CrossRef]
- Rengier, F.; Mehndiratta, A.; von Tengg-Kobligk, H.; Zechmann, C.M.; Unterhinninghofen, R.; Kauczor, H.U.; Giesel, F.L. 3D printing based on imaging data: Review of medical applications. Int. J. Comput. Assist. Radiol. Surg. 2010, 5, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, N.; Mashiko, T.; Ohnishi, T.; Ohta, M.; Namba, K.; Watanabe, E.; Kawai, K. Manufacture of patient-specific vascular replicas for endovascular simulation using fast, low-cost method. Sci. Rep. 2016, 6, 39168. [Google Scholar] [CrossRef] [PubMed]
- Haissaguerre, M.; Gencel, L.; Fischer, B.F.; Métayer, P.; Poquet, F.; Marcus, F.I.; Clementy, J. Successful catheter ablation of atrial fibrillation. J. Cardiovasc. Electrophysiol. 1994, 5, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.T.; Barham, W.; Zheng, L.; Dinegar, S.; Tzou, W.S.; Sauer, W.H. Effect of radiofrequency energy delivery in proximity to metallic medical device components. Heart Rhythm 2015, 12, 2162–2169. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.K.; Fabre, A.; Cunningham, M.; Buckley, U.; Crotty, T.; Keane, D. Post mortem study of the depth and circumferential location of sympathetic nerves in human renal arteries—Implications for renal denervation catheter design. Catheter. Cardiovasc. Interv. 2015, 86, E32–E37. [Google Scholar] [CrossRef] [PubMed]
- Dow Corning Corp. Available online: http://www.dowcorning.com/applications/search/default.aspx?r=131en (accessed on 18 August 2017).
- Cohen, M.L. Measurement of the thermal properties of human skin. Rev. J. Investig. Dermatol. 1977, 69, 333–338. [Google Scholar] [CrossRef]
- Maruyama, H.; Otake, T.; Arai, F. Photoprocessible Hydrogel Microsensor for Local Environment Measurement on a Microfluidic Chip. IEEE/ASME Trans. Mechatron. 2011, 16, 845–852. [Google Scholar] [CrossRef]
- Tosco Co., Ltd. Available online: http://www.tosco-intl.co.jp/Irreversible_Thermochomic_color.pdf (accessed on 18 August 2017).


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayakawa, T.; Maruyama, H.; Watanabe, T.; Arai, F. Three-Dimensional Blood Vessel Model with Temperature-Indicating Function for Evaluation of Thermal Damage during Surgery. Sensors 2018, 18, 345. https://doi.org/10.3390/s18020345
Hayakawa T, Maruyama H, Watanabe T, Arai F. Three-Dimensional Blood Vessel Model with Temperature-Indicating Function for Evaluation of Thermal Damage during Surgery. Sensors. 2018; 18(2):345. https://doi.org/10.3390/s18020345
Chicago/Turabian StyleHayakawa, Takeshi, Hisataka Maruyama, Takafumi Watanabe, and Fumihito Arai. 2018. "Three-Dimensional Blood Vessel Model with Temperature-Indicating Function for Evaluation of Thermal Damage during Surgery" Sensors 18, no. 2: 345. https://doi.org/10.3390/s18020345





