Methionine-Capped Gold Nanoclusters as a Fluorescence-Enhanced Probe for Cadmium(II) Sensing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Instruments
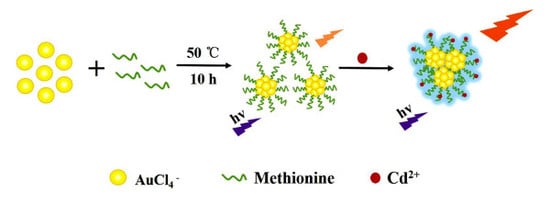
2.3. Synthesis of Au NCs
2.4. Fluorescent Detection of Cd2+
2.5. Selectivity Measurement
2.6. Analysis of Real Samples
3. Results and Discussion
3.1. Characterization of Au NCs
3.2. Optimization of Sensing Conditions
3.3. Au NCs Fluorescent Sensing of Cd2+
3.4. Selectivity of the Detection System
3.5. Mechanism of the Sensing System
3.6. Real Sample Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shi, Z.; Han, Q.; Yang, L.; Yang, H.; Tang, X.; Dou, W.; Li, Z.; Zhang, Y.; Shao, Y.; Guan, L.; et al. A highly selective two-photon fluorescent probe for detection of cadmium(II) based on intramolecular electron transfer and its imaging in living cells. Chemistry 2015, 21, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Miao, Q.; Wu, Z.; Hai, Z.; Tao, C.; Yuan, Q.; Gong, Y.; Guan, Y.; Jiang, J.; Liang, G. Bipyridine hydrogel for selective and visible detection and absorption of Cd2+. Nanoscale 2015, 7, 2797–2804. [Google Scholar] [CrossRef] [PubMed]
- Anthemidis, A.N. Flow injection on-line hydrophobic sorbent extraction for flame atomic absorption spectrometric determination of cadmium in water samples. Microchim. Acta 2008, 160, 455–460. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, Z.; Zheng, H.; Jin, L.; Hu, S.H. Significant signal enhancement of dielectric barrier discharge plasma induced vapor generation by using non-ionic surfactants for determination of mercury and cadmium by atomic fluorescence spectrometry. J. Anal. At. Spectrom. 2015, 31, 383–389. [Google Scholar] [CrossRef]
- Davis, A.C.; Calloway, C.P., Jr.; Jones, B.T. Direct determination of cadmium in urine by tungsten-coil inductively coupled plasma atomic emission spectrometry using palladium as a permanent modifier. Talanta 2007, 71, 1144–1149. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.D.; Yin, J.H.; Pu, G.; Yuan, Y.; Meng, L.; Xu, N. A simple polyethylenimine-salicylaldehyde fluorescence probe: Sensitive and selective detection of Zn2+ and Cd2+ in aqueous solution by adding S2− ion. Chem. Phys. Lett. 2016, 666, 68–72. [Google Scholar] [CrossRef]
- Dai, Y.; Yao, K.; Fu, J.; Xue, K.; Yang, L.; Xu, K. A novel 2-(hydroxymethyl)quinolin-8-ol-based selective and sensitive fluorescence probe for Cd2+ ion in water and living cells. Sens. Actuators B 2017, 251, 877–884. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, C.; He, W.; Yang, Z.; Gao, X.; Guo, Z. A highly sensitive ratiometric fluorescent probe for Cd2+ detection in aqueous solution and living cells. Chem. Commun. 2010, 46, 6138–6140. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.B.; Mondal, J.; Khudabukhsh, A.R.; Rajak, K.K. A novel fluorene based “turn on” fluorescent sensor for the determination of zinc and cadmium: Experimental and theoretical studies along with live cell imaging. New J. Chem. 2016, 40, 9593–9608. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, X.; Zheng, M.; Yang, R.; Yang, H.; Jia, L.; Yang, M. A 4,5-quinolimide-based fluorescent sensor for the turn-on detection of Cd2+ with live-cell imaging. Org. Biomol. Chem. 2017, 15, 2211–2216. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Li, R.F.; Xing, S.K.; Liu, X.M.; Hu, T.L.; Bu, X.H. A highly selective on/off fluorescence sensor for cadmium(II). Inorg. Chem. 2011, 50, 10041–10046. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Li, G.; Ren, Y.Y.; Huang, H.; Wen, X.; Xu, Q.; Fan, X.; Huang, Z.; Huang, J.; Xu, L. A selective fluorescent probe for the detection of Cd2+ in different buffer solutions and water. Dalton Trans. 2016, 45, 12087–12093. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-B.; Gu, W.; Huang, H.-X.; Wang, J.-B.; Shen, W.-X.; Lv, Y.-Y.; Shen, J. A porphyrin-based fluorescent probe for optical detection of toxic Cd2+ ion in aqueous solution and living cells. Dyes Pigments 2017, 143, 427–435. [Google Scholar] [CrossRef]
- Brahim, N.B.; Mohamed, N.B.H.; Echabaane, M.; Haouari, M.; Chaâbane, R.B.; Negrerie, M.; Ouada, H.B. Thioglycerol-functionalized CdSe quantum dots detecting cadmium ions. Sens. Actuators B 2015, 220, 1346–1353. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.; Kar, S.; Santra, S. A simple strategy for quantum dot assisted selective detection of cadmium ions. Chem. Commun. 2008, 26, 3037–3039. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Wu, W.; Deng, F.; Chen, D.; Luo, S.; Au, C. Quantum dot-based turn-on fluorescent probe for imaging intracellular zinc(II) and cadmium(II) ions. Microchim. Acta 2014, 181, 1361–1367. [Google Scholar] [CrossRef]
- Wu, Q.; Zhou, M.; Shi, J.; Li, Q.; Yang, M.; Zhang, Z. Synthesis of water-soluble Ag2S quantum dots with fluorescence in the second near-infrared window for turn-on detection of Zn(II) and Cd(II). Anal. Chem. 2017, 89, 6616–6623. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.Y.; Lin, L.P.; Rong, M.C.; Jiang, Y.Q.; Chen, X. Silver-gold alloy nanoclusters as a fluorescence-enhanced probe for aluminum ion sensing. Anal. Chem. 2013, 85, 9839–9844. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Dong, S.; Nienhaus, G.U. Ultra-small fluorescent metal nanoclusters: Synthesis and biological applications. Nano Today 2011, 6, 401–418. [Google Scholar] [CrossRef]
- Xu, H.; Suslick, K.S. Water-soluble fluorescent silver nanoclusters. Adv. Mater. 2010, 22, 1078–1082. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chen, W. Sub-nanometre sized metal clusters: From synthetic challenges to the unique property discoveries. Chem. Soc. Rev. 2012, 41, 3594–3623. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Luo, Z.; Yu, Y.; Yao, Q.; Xie, J. Luminescent noble metal nanoclusters as an emerging optical probe for sensor development. Chem. Asian J. 2013, 8, 858–871. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, V.; Shukla, A.; Sivakumar, S.; Verma, S. Purine-stabilized green fluorescent gold nanoclusters for cell nuclei imaging applications. ACS Appl. Mater. Interfaces 2014, 6, 2185–2191. [Google Scholar] [CrossRef] [PubMed]
- Knoblauch, C.; Griep, M.; Friedrich, C. Recent advances in the field of bionanotechnology: An insight into optoelectric bacteriorhodopsin, quantum dots, and noble metal nanoclusters. Sensors 2014, 14, 19731–19766. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.J.; Shan, D.; Zhu, R.H.; Deng, S.Y.; Cosnier, S.; Zhang, X.J. Dumbbell-shaped carbon quantum dots/auncs nanohybrid as an efficient ratiometric fluorescent probe for sensing cadmium(II) ions and l-ascorbic acid. Carbon 2016, 96, 1034–1042. [Google Scholar] [CrossRef]
- Naaz, S.; Chowdhury, P. Sunlight and ultrasound-assisted synthesis of photoluminescent silver nanoclusters: A unique ‘knock out’ sensor for thiophilic metal ions. Sens. Actuators B 2017, 241, 840–848. [Google Scholar] [CrossRef]
- Li, J.; Zhong, X.; Zhang, H.; Le, X.C.; Zhu, J.J. Binding-induced fluorescence turn-on assay using aptamer-functionalized silver nanocluster DNA probes. Anal. Chem. 2012, 84, 5170–5174. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Liu, B.; Jin, W.; Wu, F.; Wan, Y. Colorimetric detection of Cd2+ using 1-amino-2-naphthol-4-sulfonic acid functionalized silver nanoparticles. J. Nanopart. Res. 2016, 18, 327–336. [Google Scholar] [CrossRef]
- Maruyama, T.; Fujimoto, Y.; Maekawa, T. Synthesis of gold nanoparticles using various amino acids. J. Colloid Interface Sci. 2015, 447, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zhou, C.; Yu, M.; Liu, J. Different sized luminescent gold nanoparticles. Nanoscale 2012, 4, 4073–4083. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Dorlich, R.M.; Brandholt, S.; Schneider, R.; Trouillet, V.; Bruns, M.; Gerthsen, D.; Nienhaus, G.U. Facile preparation of water-soluble fluorescent gold nanoclusters for cellular imaging applications. Nanoscale 2011, 3, 2009–2014. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Luo, Y.; Zhuo, Y.; Feng, Y.; Zhu, S. Novel synthesis of gold nanoclusters templated with l-tyrosine for selective analyzing tyrosinase. Anal. Chim. Acta 2014, 840, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Qi, L.; Qiao, J.; Ma, H. One-pot synthesis of tyrosine-stabilized fluorescent gold nanoclusters and their application as turn-on sensors for Al3+ ions and turn-off sensors for Fe3+ ions. Anal. Methods 2014, 6, 6445–6451. [Google Scholar] [CrossRef]
- Liu, C.L.; Wu, H.T.; Hsiao, Y.H.; Lai, C.W.; Shih, C.W.; Peng, Y.K.; Tang, K.C.; Chang, H.W.; Chien, Y.C.; Hsiao, J.K. Insulin-directed synthesis of fluorescent gold nanoclusters: Preservation of insulin bioactivity and versatility in cell imaging. Angew. Chem. 2011, 50, 7056–7060. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Azadfar, N.; Stockmar, F.; Send, W.; Trouillet, V.; Bruns, M.; Gerthsen, D.; Nienhaus, G.U. One-pot synthesis of near-infrared fluorescent gold clusters for cellular fluorescence lifetime imaging. Small 2011, 7, 2614–2620. [Google Scholar] [CrossRef] [PubMed]
- Whetten, R.L.; Price, R.C. Nano-golden order. Science 2007, 318, 407–408. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.F.; Wang, Y.S.; Zhou, B.; Yu, J.H.; Peng, L.L.; Huang, Y.Q.; Li, X.J.; Chen, S.H.; Tang, X.; Wang, X.F. A multifunctional fluorescent aptamer probe for highly sensitive and selective detection of cadmium(II). Anal. Bioanal. Chem. 2017, 409, 4951–4958. [Google Scholar] [CrossRef] [PubMed]
- Aragay, G.; Pons, J.; Merkoci, A. Recent trends in macro-, micro-, and nanomaterial-based tools and strategies for heavy-metal detection. Chem. Rev. 2011, 111, 3433–3458. [Google Scholar] [CrossRef] [PubMed]







| Sample | Detected (µM) | Spiked (µM) | Found (µM) | Recovery (%) | RSD (%) (n = 3) | ICP-OES (µM) |
|---|---|---|---|---|---|---|
| Tap water | ND 1 | 3.00 | 2.89 | 96.33 | 2.56 | 2.97 |
| 5.00 | 5.10 | 101.95 | 3.56 | 5.07 | ||
| 10.00 | 10.62 | 106.21 | 1.45 | 10.08 | ||
| Lake water | ND 1 | 3.00 | 2.86 | 95.33 | 2.37 | 3.02 |
| 5.00 | 4.84 | 96.74 | 2.15 | 5.06 | ||
| 10.00 | 10.41 | 104.14 | 0.51 | 10.08 | ||
| Milk powders | ND 1 | 3.00 | 2.96 | 98.66 | 2.56 | 2.92 |
| 5.00 | 5.06 | 101.29 | 1.68 | 5.03 | ||
| 10.00 | 10.43 | 104.32 | 1.56 | 10.05 | ||
| Camel milk powders | ND 1 | 3.00 | 2.87 | 95.67 | 2.79 | 2.95 |
| 5.00 | 4.96 | 99.23 | 2.31 | 5.04 | ||
| 10.00 | 10.26 | 102.62 | 1.87 | 10.09 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Y.; Wang, M.; Wu, X.; Wang, F.; Liu, L. Methionine-Capped Gold Nanoclusters as a Fluorescence-Enhanced Probe for Cadmium(II) Sensing. Sensors 2018, 18, 658. https://doi.org/10.3390/s18020658
Peng Y, Wang M, Wu X, Wang F, Liu L. Methionine-Capped Gold Nanoclusters as a Fluorescence-Enhanced Probe for Cadmium(II) Sensing. Sensors. 2018; 18(2):658. https://doi.org/10.3390/s18020658
Chicago/Turabian StylePeng, Yan, Maomao Wang, Xiaoxia Wu, Fu Wang, and Lang Liu. 2018. "Methionine-Capped Gold Nanoclusters as a Fluorescence-Enhanced Probe for Cadmium(II) Sensing" Sensors 18, no. 2: 658. https://doi.org/10.3390/s18020658