Laser Spectroscopic Sensors for the Development of Anthropomorphic Robot Sensitivity
Abstract
:1. Introduction
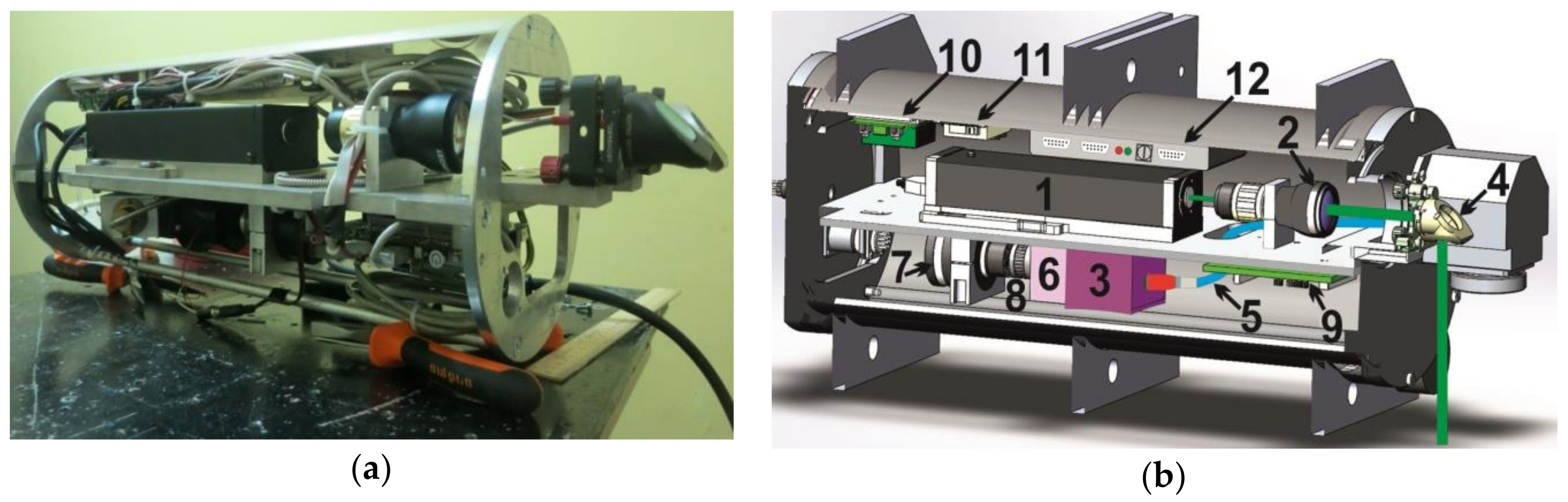
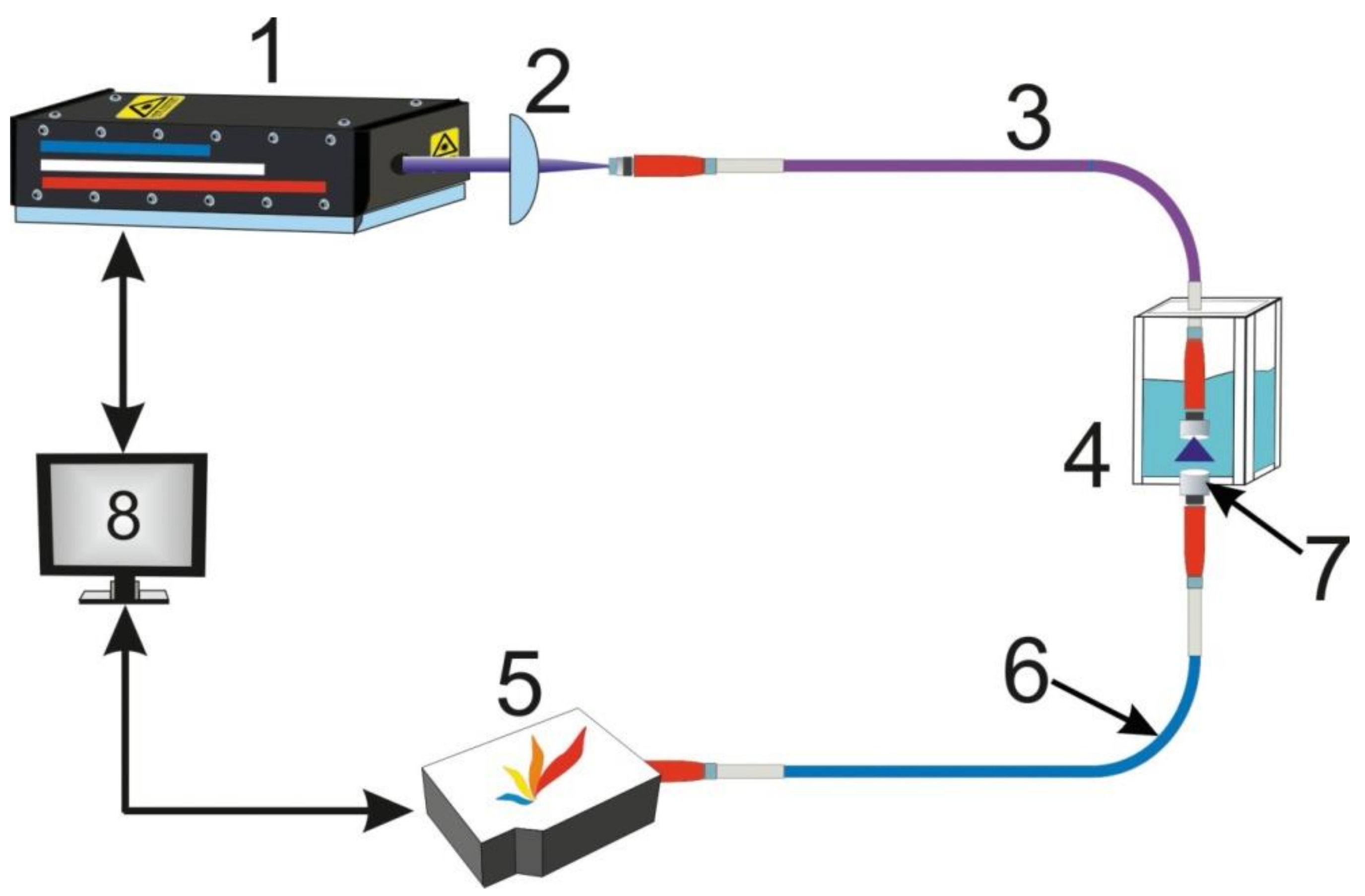
2. Instrument Design
3. Materials and Methods
3.1. LIF Sensor Development
3.2. LIBS Sensor Development
4. Results
4.1. LIF
- −
- the intensity values of the LIF spectrums, which were excited by femtosecond laser, turned out to be small for each solution;
- −
- a relative spectral distribution of the LIF is the same for both the femtosecond and nanosecond LIF;
- −
- specific wide maximums were marked for different fractions of oil and this might be used to identify it; both of these wide maximums are within the spectrum of crude oil.
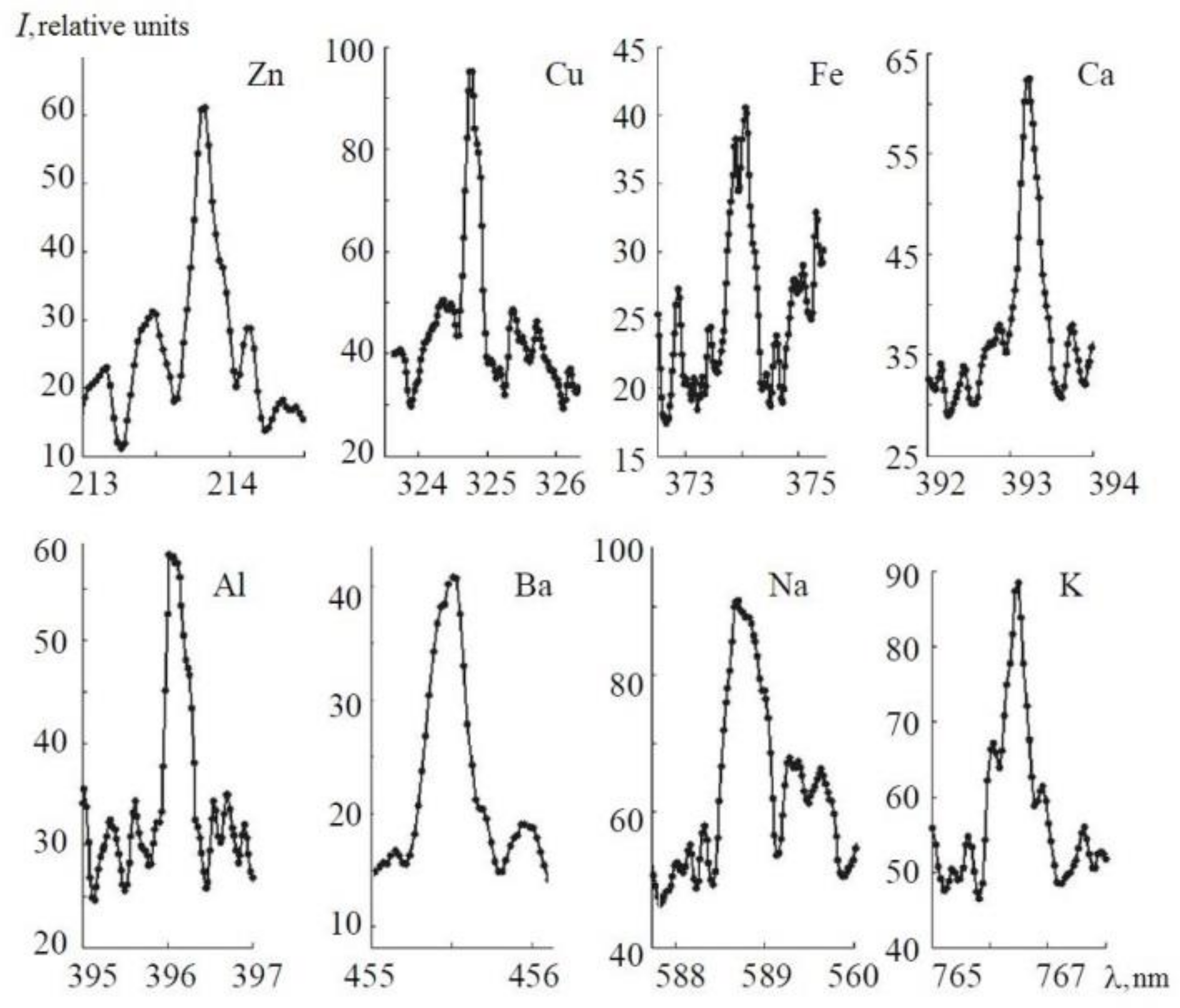
4.2. LIBS
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schill, F.; Zimmer, U.R.; Trumpf, J. Visible spectrum optical communication and distance sensing for underwater applications. In Proceedings of the ACRA 2004, Canberra, Australia, 6–8 December 2004. [Google Scholar]
- The Marine Technology Specialists. Available online: http://seatronics-group.com/equipment-rental/diving-ndt/subsea-cameras/tritech-typhoon-vms/ (accessed on 2 April 2018).
- 2G Robotics|Underwater Laser Scanners and Imaging Solutions. Available online: http://www.2grobotics.com/products/underwater-laser-scanner-uls-100/ (accessed on 2 April 2018).
- Raymond, M. Laser Remote Sensing: Fundamentals and Application; Krieger Publishing Company: Malabar, FL, USA, 1984. [Google Scholar]
- Gereit, F.; Hauptmann, P.; Matz, G.; Mellert, V.; Reuter, R. An ROV-based sensor system for maritime pollution control. In Proceedings of the Oceanology International 98 Conference, Brighton, UK, 10–13 March 1998. [Google Scholar]
- Brewer, P.G.; Malby, G.; Pasteris, J.; White, S.; Peltzer, E.; Wopenka, B.; Brown, M. Development of a laser Raman spectrofluorometer for deep-ocean science. Deep Sea Res. Part I Oceanogr. Res. Pap. 2004, 51, 739–753. [Google Scholar] [CrossRef]
- Sherman, A.D.; Walz, P.M.; Brewer, P.G. Two Generation of Deep-Ocean Raman In-Situ Spectrofluorometers. Sea Technol. 2007, 2, 10–13. [Google Scholar]
- Puiu, A.; Fiorani, L.; Menicucci, I.; Pistilli, M.; Lai, A. Submersible spectrofluorometer for real-time sensing of water quality. Sensors 2015, 15, 14415–14434. [Google Scholar] [CrossRef] [PubMed]
- Hussein, A.; Marzouk, A. Characterization of petroleum crude oils using Laser Induced Fluorescence. J. Pet. Environ. Biotechnol. 2015, 6, 3–6. [Google Scholar] [CrossRef]
- Damon, E.K.; Tomlinson, R.G. Observation of ionization of gases by a ruby laser. Appl. Opt. 1963, 2, 546–547. [Google Scholar] [CrossRef]
- Lin, Q.-Y.; Duan, Y.-X. Laser-Induced Breakdown Spectroscopy: From Experimental Platform to Field Instrument. Chin. J. Anal. Chem. 2017, 45, 1405–1414. [Google Scholar] [CrossRef]
- Thornton, B.; Takahashi, T.; Sato, T.; Sakka, T.; Tamura, A.; Matsumoto, A.; Nozaki, T.; Ohki, T.; Ohki, K. Development of a deep-sea laser-induced breakdown spectrofluorometer for in situ multi-element chemical analysis. Deep Sea Res. Part I: Oceanogr. Res. Pap. 2015, 95, 20–36. [Google Scholar] [CrossRef]
- Golik, S.S.; Ilyin, A.A.; Babiy, M. Yu.; Biryukova, Yu. S.; Lisitsa, V.V.; Bukin, O.A. Determination of Iron in Water Solution by Time-Resolved Femtosecond Laser-Induced Breakdown Spectroscopy. Plasma Sci. Technol. 2015, 17, 975–978. [Google Scholar] [CrossRef]
- Goueguel, C.; McIntyre, D.L.; Singh, J.P.; Jain, J.; Karamalidis, A.K. Laser-Induced Breakdown Spectroscopy (LIBS) of a High-Pressure CO2–Water Mixture: Application to Carbon Sequestration. Appl. Spectrosc. 2014, 68, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Goueguel, C.; Mcintyre, D.L.; Jain, J.; Karamalidis, A.K.; Carson, C. Matrix effect of sodium compounds on the determination of metal ions in aqueous solutions by underwater laser-induced breakdown spectroscopy. Appl. Opt. 2015, 54, 6071–6079. [Google Scholar] [CrossRef] [PubMed]
- Gaudiuso, R.; Dell’Aglio, M.; Pascale, O.D.; Senesi, G.S.; Giacomo, A.D. Laser induced breakdown spectroscopy for elemental analysis in environmental, cultural heritage and space applications: A review of methods and results. Sensors 2010, 10, 7434–7468. [Google Scholar] [CrossRef] [PubMed]
- Hahn, D.W.; Omenetto, N. Laser-induced breakdown spectroscopy (LIBS), part I: Review of basic diagnostics and plasma–particle interactions: Still-challenging issues within the analytical plasma community. Appl. Spectrosc 2010, 64, 335A–366A. [Google Scholar] [CrossRef] [PubMed]
- Hahn, D.W.; Omenetto, N. Laser-induced breakdown spectroscopy (LIBS), part II: Review of instrumental and methodological approaches to material analysis and applications to different fields. Appl. Spectrosc. 2012, 66, 347–419. [Google Scholar] [CrossRef] [PubMed]
- López-Claros, M.; Fortes, F.J.; Laserna, J.J. Subsea spectral identification of shipwreck objects using laser-induced breakdown spectroscopy and linear discriminant analysis. J. Cult. Herit. 2017, 29, 75–81. [Google Scholar] [CrossRef]
- Moros, J.; Laserna, J.J. Unveiling the identity of distant targets through advanced Raman-laser-induced breakdown spectroscopy data fusion strategies. Talanta 2015, 134, 627–639. [Google Scholar] [CrossRef] [PubMed]
- Musazzi, S.; Perini, U. Laser-Induced Breakdown Spectroscopy; Springer Series in Optical Sciences; Springer: New York, NY, USA, 2014; p. 182. [Google Scholar]
- Lazic, V.; Laserna, J.J.; Jovicevic, S. Insights in the laser-induced breakdown spectroscopy signal generation underwater using dual pulse excitation—Part I: Vapor bubble, shockwaves and plasma. Spectrochim. Acta Part B At. Spectrosc. 2013, 82, 42–49. [Google Scholar] [CrossRef]
- Guirado, S.; Fortes, F.J.; Laserna, J.J. Multi-pulse excitation for underwater analysis of copper-based alloys using a novel remote laser-induced breakdown spectroscopy (LIBS) system. Appl. Spectrosc. 2016, 70, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Bukin, O.A.; Alekseev, A.V.; Ilyin, A.A.; Golik, S.S.; Tsarev, V.I.; Bodin, N.S. Application of laser induced breakdown spectroscopy with multipulse plasma generation to monitoring seawater quality and the state of phytoplankton. Atmos. Ocean. Opt. 2003, 16, 20–25. [Google Scholar]
- Bukin, O.A.; Mayor, A.Y.; Proschenko, D.Y.; Bukin, I.O.; Bolotov, V.V.; Chekhlenok, A.A.; Mun, S.A. Laser spectroscopy methods in the development of laser sensor elements for underwater robotics. Atmos. Ocean. Opt. 2017, 30, 475–480. [Google Scholar] [CrossRef]
- Golik, S.S.; Bukin, O.A.; Ilyin, A.A.; Sokolova, E.B.; Kolesnikov, A.V.; Babiy, M. Yu.; Kulchin, Yu. N.; Galchenko, A.A. Determination of detection limits for elements in water by femtosecond laser-induced breakdown spectroscopy. J. Appl. Spectrosc. 2012, 79, 471–476. [Google Scholar] [CrossRef]
- Cremers, D.A.; Radziemski, L.J. Handbook of Laser-Induced Breakdown Spectroscopy; John Wiley & Sons, Ltd.: New York, NY, USA, 2006. [Google Scholar]
- Yueh, F.Y.; Sharma, R.C.; Singh, J.P.; Zhang, H.; Spencer, W. Evaluation of the Potential of Laser-Induced Breakdown Spectroscopy for Detection of Trace Element in Liquid. J. Air Waste Manag. Assoc. 2002, 52, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Y.; Hao, Z.Q.; Li, C.M.; Li, J.M.; Yi, R.X.; Shen, M.; Zeng, X.Y. Sensitive determinations of Cu, Pb, Cd, and Cr elements in aqueous solutions using chemical replacement combined with surface-enhanced laser-induced breakdown spectroscopy. Opt. Express 2016, 24, 13410–13417. [Google Scholar] [CrossRef] [PubMed]
- Xiu, J.; Zhong, S.; Hou, H.; Lu, Y.; Zheng, R. Quantitative determination of manganese in aqueous solutions and seawater by laser-induced breakdown spectroscopy (LIBS) using paper substrates. Appl. Spectrosc. 2014, 68, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Turekian, K.K. Oceans; Prentice-Hall: Englewood Cliffs, NJ, USA, 1968. [Google Scholar]
- Aras, N.; Yesiller, S.U.; Ates, D.A.; Yalcin, S. Ultrasonic nebulization-sample introduction system for quantitative analysis of liquid samples by laser-induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2012, 74, 87–94. [Google Scholar] [CrossRef]
- Fichet, P.; Mauchien, P.; Wagner, J.F.; Moulin, C. Quantitative elemental determination in water and oil by laser induced breakdown spectroscopy. Anal. Chim. Acta 2001, 429, 269–278. [Google Scholar] [CrossRef]
- Cahoon, E.M.; Almirall, J.R. Quantitative Analysis of Liquids from Aerosols and Microdrops Using Laser Induced Breakdown Spectroscopy. Anal. Chem. 2012, 84, 2239–2244. [Google Scholar] [CrossRef] [PubMed]
- Sobral, H.; Sanginés, R.; Trujillo-Vázquez, A. Detection of trace elements in ice and water by laser-induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2012, 78, 62–66. [Google Scholar] [CrossRef]
- Skočovská, K.; Novotný, J.; Prochazka, D.; Pořízka, P.; Novotný, K.; Kaiser, J. Optimization of liquid jet system for laser-induced breakdown spectroscopy analysis. Rev. Sci. Instrum. 2016, 87, 043116. [Google Scholar] [CrossRef] [PubMed]
- Xiu, J.; Dong, L.; Qin, H.; Liu, Y.; Yu, J. Investigation of the Matrix Effect on the Accuracy of Quantitative Analysis of Trace Metals in Liquids Using Laser-Induced Breakdown Spectroscopy with Solid Substrates. Appl. Spectrosc. 2016, 70, 2016–2024. [Google Scholar] [CrossRef] [PubMed]









| Type of Oil Products | LoD (%), ns | LoD (%), fs |
|---|---|---|
| 1. Crude oil | 0.018 | 0.047 |
| 2. RMB (Residual Marine B)-30 | 0.027 | 0.049 |
| 3. DM (Distillate Marine) A | 0.0003 | 0.002 |
| Element | Wavelength, Our Work (nm) | Gate Delay, Our Work (ns) | Repetition Rate (Hz) | LOD, Our Data (ppm, fs) | Literature Data (ppm, ns) |
| AlI | 396.1 | 130 | 50 | 0.19 | 2.7 [32], 10 [33] |
| Ca I | 393.3 | 55 | 50 | 0.01 | 0.3 [33], 0.94 [15], 1.4 [32], 0.6 [34] |
| Cu | 324.7 | 92 | 100 | 0.78 | 7 [33], 0.25 [29], 9.6 [35], 12 [36], 0.39 [30], 1.67 [37], 1.7 [32] |
| Pb | 283 | 130 | 150 | 3.8 | 1.27 [30], 0.13 [29], 13 [36], 12.5 [35], 13.6 [32] |
| Zn | 213.8 | 57 | 100 | 2.5 | 0.51 [30], 41.6 [32] |
| Ba I | 455.4 | 72 | 100 | 0.08 | 0.7 [33] |
| Fe | 371.9 | 160 | 50 | 2.6 | 30 [33], 10.5 [35] |
| K | 766.5 | 180 | 100 | 0.006 | 0.03 [15], 6.01 [32] |
| Mg I | 279.5 | 130 | 150 | 0.26 | 1 [32], 0.9 [35], 0.1 [28], 1.0 [33], 0.3 [34] |
| Na | 588.9 | 210 | 100 | 0.0009 | 0.5 [33], 0.45 [32] |
| Sr | 460.7 | 130 | 150 | 0.44 | 2.89 [15], 1 [34] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bukin, O.; Proschenko, D.; Chekhlenok, A.; Golik, S.; Bukin, I.; Mayor, A.; Yurchik, V. Laser Spectroscopic Sensors for the Development of Anthropomorphic Robot Sensitivity. Sensors 2018, 18, 1680. https://doi.org/10.3390/s18061680
Bukin O, Proschenko D, Chekhlenok A, Golik S, Bukin I, Mayor A, Yurchik V. Laser Spectroscopic Sensors for the Development of Anthropomorphic Robot Sensitivity. Sensors. 2018; 18(6):1680. https://doi.org/10.3390/s18061680
Chicago/Turabian StyleBukin, Oleg, Dmitriy Proschenko, Alexey Chekhlenok, Sergey Golik, Ilya Bukin, Alexander Mayor, and Victoriya Yurchik. 2018. "Laser Spectroscopic Sensors for the Development of Anthropomorphic Robot Sensitivity" Sensors 18, no. 6: 1680. https://doi.org/10.3390/s18061680




