Polystyrene Oxygen Optodes Doped with Ir(III) and Pd(II) meso-Tetrakis(pentafluorophenyl)porphyrin Using an LED-Based High-Sensitivity Phosphorimeter
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Oxygen Optodes
2.2. Sensor Characterization
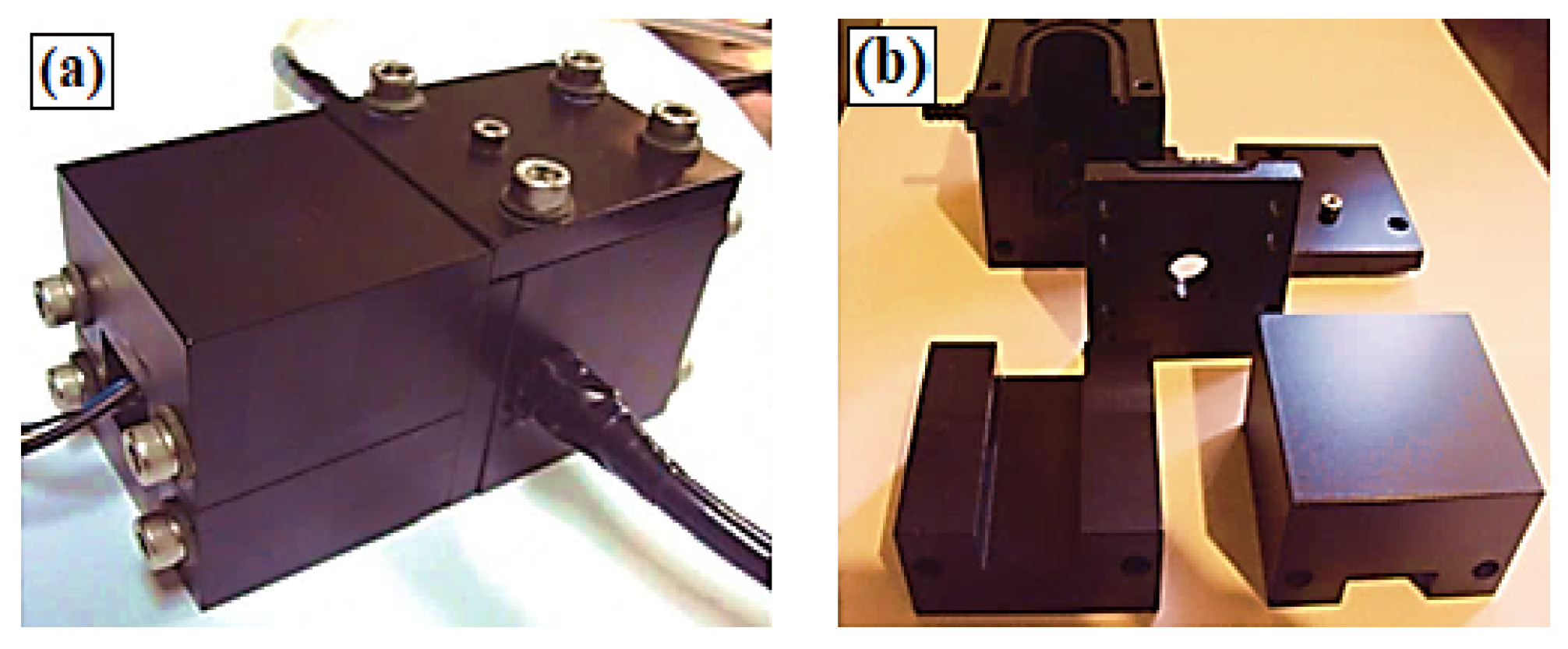
2.2.1. Instrument Setup
Pulsed Light Source (Figure 2b, No.1)
Test Chamber (Figure 2b, No.2)
Emission Filter (Figure 2b, No.3)
Photomultiplier Tube (Figure 2b, No.4)
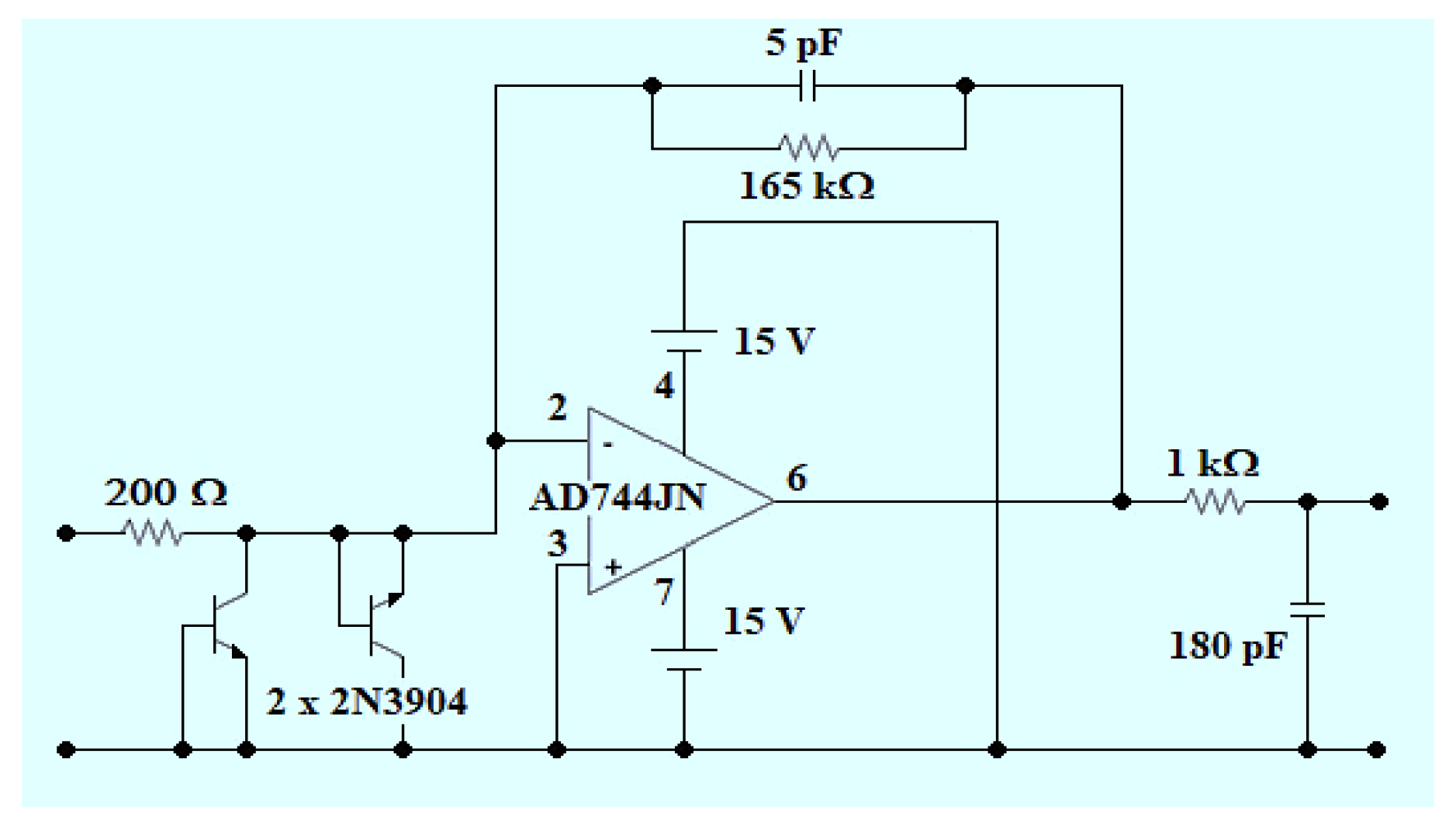
Conditioning of the Phosphorescent Signal (Figure 2b, No.5)
Lifetime Acquisition and Processing (Figure 2b, No.6)
2.3. Lifetime and Photostability Measurements
3. Results and Discussion
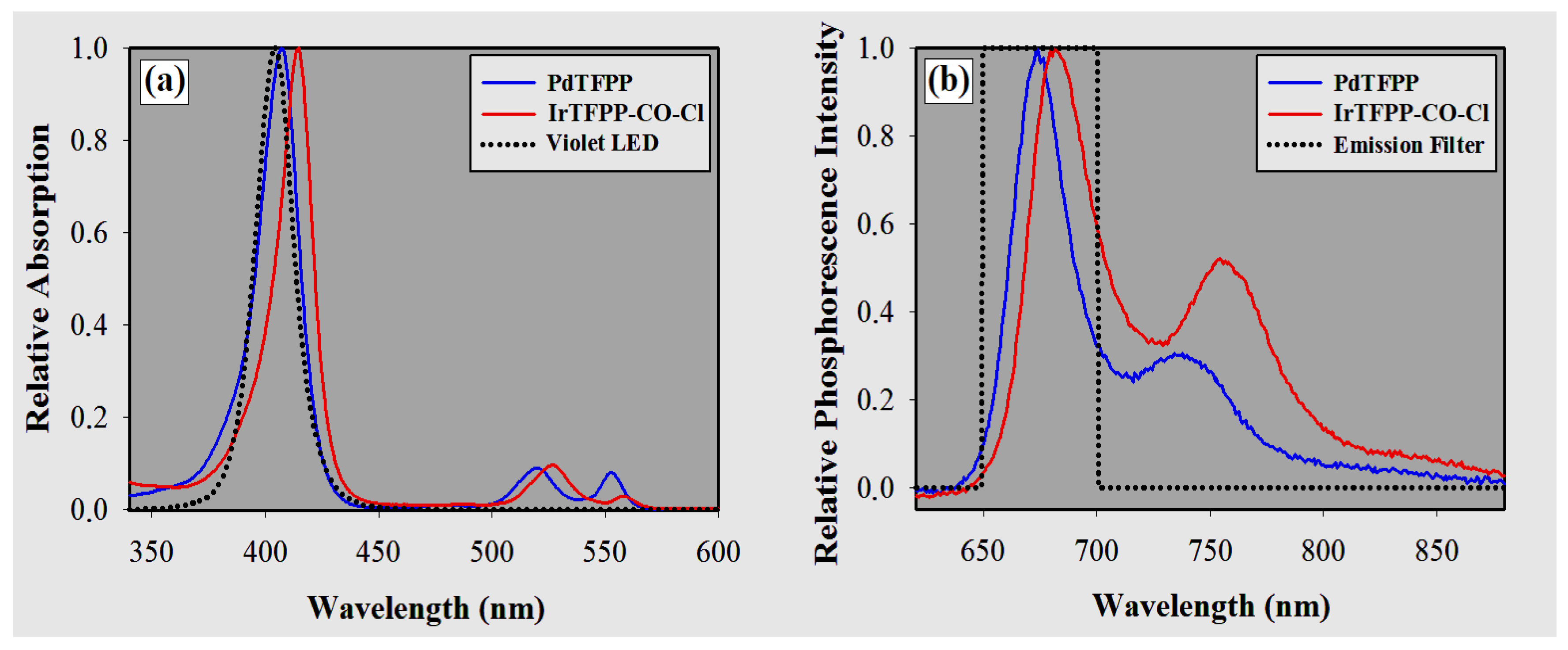
3.1. Spectra Measurements
3.2. Residual Excitation Light
3.3. Sensitivity of the Oxygen Optodes
3.3.1. Brightness
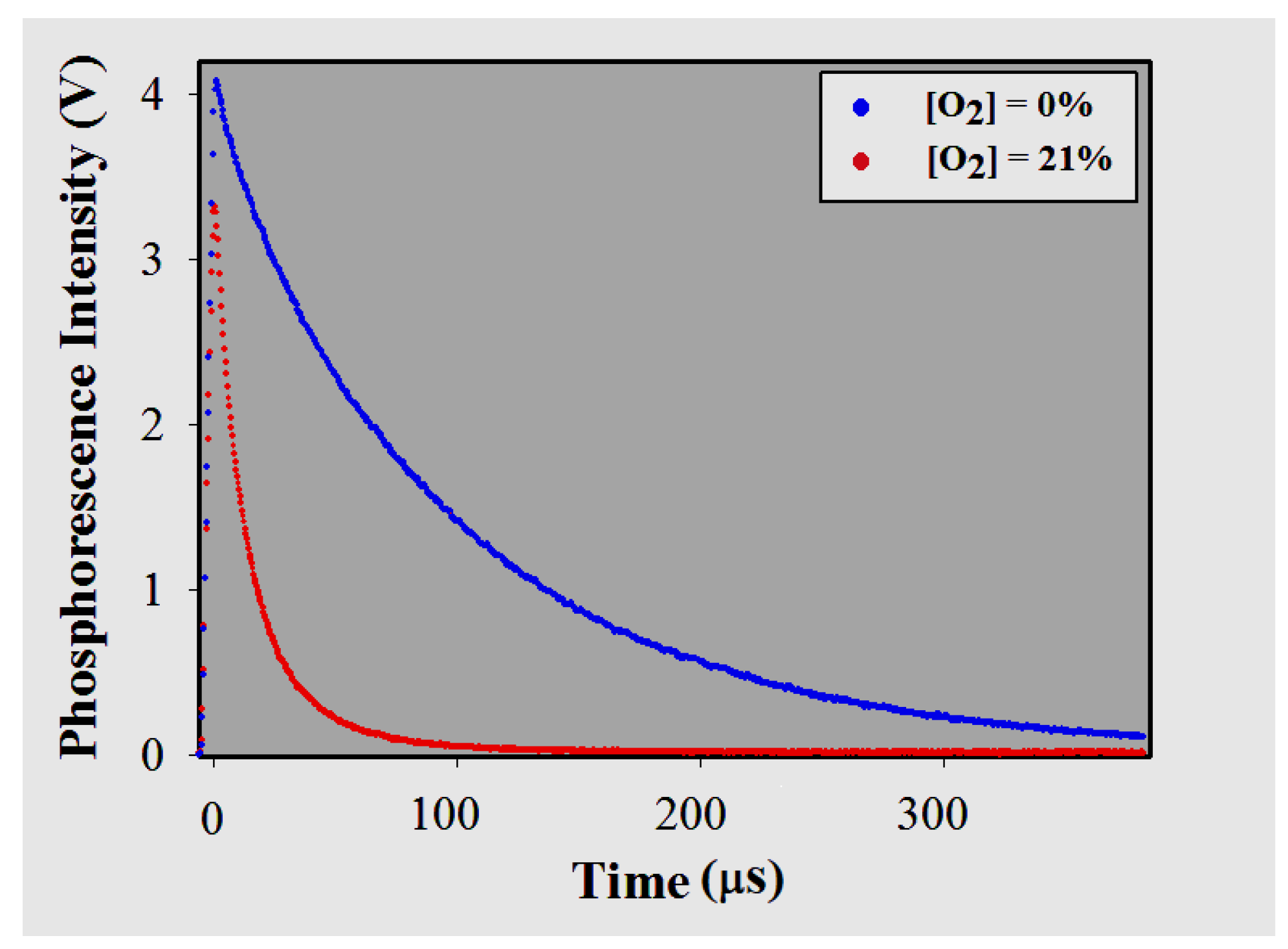
3.3.2. Phosphorescence Lifetimes
3.3.3. Stern–Volmer Plots
3.4. Photostability
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Ir-OEP-CO-Cl | Chloro(octaethylporphyrinato)-carbonyl iridium(III) |
| IrTFPP-CO-Cl | Chloro(meso-tetrakis(pentafluorophenyl)porphyrinato)-carbonyl iridium(III) |
| fwhm | Full width at half maximum |
| LabVIEW | Laboratory virtual instrument engineering workbench |
| LED | Light emitting diode |
| OEP | Octaethylporphyrin |
| OLEDs | Organic light emitting diodes |
| PdPs | Palladium porphyrins |
| PdTFPP | Palladium(II) meso-tetrakis(pentafluorophenyl)porphyrin |
| PdTPTBPF | Palladium(II) meso-tetrakis(4-fluorophenyl)tetrabenzoporphyrin |
| PMT | Photomultiplier tube |
| PS | Polystyrene |
| PtPs | Platinum porphyrins |
| PtTFPP | Platinum(II) meso-tetrakis(pentafluorophenyl)porphyrin |
| Ru(dpp) 2 | Ruthenium(II) diphenylphenanthroline |
| SNR | Signal-to-noise ratio |
| TFPP | meso-tetrakis(pentafluorophenyl)porphyrin |
| TIA | Transimpedance amplifier |
| TPP(p-COCH) | meso-tetra(para-methoxycarbonylphenyl)porphyrin |
| TPTBP | meso-tetra(phenyl)tetrabenzoporphyrin |
References
- Mathew, S.; Yella, A.; Gao, P.; Humphry-Baker, R.; Curchod, B.F.E.; Ashari-Astani, N.; Tavernelli, I.; Rothlisberger, U.; Nazeeruddin, M.K.; Gratzel, M. Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nat. Chem. 2014, 6, 242–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sommer, J.R.; Shelton, A.H.; Parthasarathy, A.; Ghiviriga, I.; Reynolds, J.R.; Schanze, K.S. Photophysical properties of near-infrared phosphorescent π-extended platinum porphyrins. Chem. Mater. 2011, 23, 5296–5304. [Google Scholar] [CrossRef]
- Quaranta, M.; Borisov, S.; Klimant, I. Indicators for optical oxygen sensors. Bioanal. Rev. 2012, 4, 115–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mills, A. Optical oxygen sensors—Utilising the luminescence of platinum metals complexes. Platin. Met. Rev. 1997, 41, 115–127. [Google Scholar]
- Ni, Y. Metalloporphyrins and functional analogues as MRI contrast agents. Curr. Med. Imaging Rev. 2008, 4, 96–112. [Google Scholar] [CrossRef]
- Roussakis, E.; Li, Z.; Nowell, N.H.; Nichols, A.J.; Evans, C.L. Bright, “clickable” porphyrins for the visualization of oxygenation under ambient light. Angew. Chem. Int. Ed. 2015, 54, 14728–14731. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.D.; Wolfbeis, O. Optical methods for sensing and imaging oxygen: Materials, spectroscopies and applications. Chem. Soc. Rev. 2014, 43, 3666–3761. [Google Scholar] [CrossRef] [PubMed]
- Rumyantseva, V.D.; Ivanovskaya, N.P.; Konovalenko, L.I.; Tsukanov, S.V.; Mironov, A.F.; Osin, N.S. Synthesis and spectral luminescent characteristics of the porphyrin complexes with the platinum group metals. Rus. J. Bioorgan. Chem. 2008, 34, 239–244. [Google Scholar] [CrossRef]
- Borisov, S.M.; Nuss, G.; Klimant, I. Red light-excitable oxygen sensing materials based on platinum(II) and palladium(II) benzoporphyrins. Anal. Chem. 2008, 80, 9435–9442. [Google Scholar] [CrossRef] [PubMed]
- Wolfbeis, O. Luminescent sensing and imaging of oxygen: Fierce competition to the Clark electrode. Bioessays 2015, 37, 921–928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, C.S.; Lo, Y.L. Ratiometric Fiber-optic oxygen sensors based on sol-gel matrix doped with metalloporphyrin and 7-amino-4-trifluoromethyl coumarin. Sens. Actuators B: Chem. 2008, 134, 711–717. [Google Scholar] [CrossRef]
- Grist, S.M.; Chrostowski, L.; Cheung, K.C. Optical oxygen sensors for applications in microfluidic cell culture. Sensors 2010, 10, 9286–9316. [Google Scholar] [CrossRef] [PubMed]
- Kostov, Y.; Rao, G. Low-cost optical instrumentation for biomedical measurements. Rev. Sci. Instrum. 2000, 71, 4361–4374. [Google Scholar] [CrossRef]
- Stich, M.I.J.; Fischer, L.H.; Wolfbeis, O.S. Multiple fluorescent chemical sensing and imaging. Chem. Soc. Rev. 2010, 39, 3102–3114. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.D.; Chen, H.X.; Zhao, Y.; Chen, X.; Wang, X.R. Optical oxygen sensors move towards colorimetric determination. Trends Anal. Chem. 2010, 29, 319–338. [Google Scholar] [CrossRef]
- Rae, B.R.; Muir, K.R.; Gong, Z.; McKendry, J.; Girkin, J.M.; Gu, E.; Renshaw, D.; Dawson, M.D.; Henderson, R.K. A CMOS time-resolved fluorescence lifetime analysis micro-system. Sensors 2009, 9, 9255–9274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mak, C.S.K.; Pentlehner, D.; Stich, M.; Wolfbeis, O.S.; Chan, W.K.; Yersin, H. Exceptional oxygen sensing capabilities and triplet state properties of Ir(ppy-NPh2)3. Chem. Mater. 2009, 21, 2173–2175. [Google Scholar] [CrossRef]
- Ast, C.; Schmalzlin, E.; Lohmannsroben, H.G.; Van Dongen, J.T. Optical oxygen micro- and nanosensors for plant applications. Sensors 2012, 12, 7015–7032. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, S.; Labuta, J.; Rossom, W.V.; Ishikawa, D.; Minami, K.; Hill, J.P.; Ariga, K. Porphyrin-based sensor nanoarchitectonics in diverse physical detection modes. Phys. Chem. Chem. Phys. 2014, 16, 9713–9746. [Google Scholar] [CrossRef] [PubMed]
- Capuano, R.; Pomarico, G.; Paolesse, R.; Di Natale, C. Corroles-porphyrins: A teamwork for gas sensor arrays. Sensors 2015, 15, 8121–8130. [Google Scholar] [CrossRef] [PubMed]
- Borisov, S.M.; Papkovsky, D.B.; Ponomarev, G.V.; DeToma, A.S.; Saf, R.; Klimant, I. Photophysical properties of the new phosphorescent platinum(II) and palladium(II) complexes of benzoporphyrins and chlorins. J. Photochem. Photobiol. A: Chem. 2009, 206, 87–92. [Google Scholar] [CrossRef]
- Niedermair, F.; Borisov, S.M.; Zenkl, G.; Hofmann, O.T.; Weber, H.; Saf, R.; Klimant, I. Tunable Phosphorescent NIR oxygen indicators based on mixed benzo- and naphthoporphyrin complexes. Inorgan. Chem. 2010, 49, 9333–9342. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.D.; Stolwijk, J.A.; Sperber, M.; Meier, R.J.; Wegener, J.; Wolfbeis, O.S. Ultra-small, highly stable, and membrane-impermeable fluorescent nanosensors for oxygen. Methods Appl. Fluoresc. 2013, 1, 035002. [Google Scholar] [CrossRef] [PubMed]
- Enko, B.; Borisov, S.M.; Regensburger, J.; Baumler, W.; Gescheidt, G.; Klimant, I. Singlet oxygen-induced photodegradation of the polymers and dyes in optical sensing materials and the effect of stabilizers on these processes. J. Phys. Chem. A 2013, 117, 8873–8882. [Google Scholar] [CrossRef] [PubMed]
- Koren, K.; Borisov, S.M.; Saf, R.; Klimant, I. Strongly phosphorescent Ir(III)-porphyrins—New oxygen indicators with tuneable photophysical properties and functionalities. Eur. J. Inorg. Chem. 2011, 10, 1531–1534. [Google Scholar] [CrossRef] [PubMed]
- Koren, K.; Dmitriev, R.I.; Borisov, S.M.; Papkovsky, D.B.; Klimant, I. Complexes of Ir(III)-octaethylporphyrin with peptides as probes for sensing cellular O2. Chembiochem 2012, 13, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.H.; Durrel, A.C.; Gross, Z.; Winkler, J.R.; Gray, H.B. Near-IR Phosphorescence of iridium(III) corroles at ambient temperature. J. Am. Chem. Soc. 2010, 132, 9230–9231. [Google Scholar] [CrossRef] [PubMed]
- Lebedev, A.Y.; Cheprakov, A.V.; Sakadzic, S.; Boas, D.A.; Wilson, D.F.; Vinogradov, S.A. Dendritic phosphorescent probes for oxygen imaging in biological systems. ACS Appl. Mater. Interfaces 2009, 1, 1292–1304. [Google Scholar] [CrossRef] [PubMed]
- Hutter, L.H.; Muller, B.J.; Koren, K.; Borisov, S.M.; Klimant, I. Robust optical oxygen sensors based on polymer-bound NIR-emitting platinum(II)-benzoporphyrins. J. Mater. Chem. C 2014, 2, 7589–7598. [Google Scholar] [CrossRef]
- Costa-Fernandez, J.M.; Bordel, N.; Campo, J.C.; Ferrero, F.J.; Perez, M.A.; Sanz-Medel, A. Portable fibre optic oxygen sensor based on room-temperature phosphorescence lifetime. Microchim. Acta 2000, 134, 145–152. [Google Scholar] [CrossRef]
- Hamamatsu Photonics K K, Electron Tube Division. Photomultiplier Tubes Modules. Available online: https://www.hamamatsu.com/resources/pdf/etd/H10720_H10721_TPMO1062E.pdf (accessed on 25 September 2015).
- Wang, T.; Erhman, B. Compensate Transimpedance Amplifiers Intuitively; Texas Instruments Inc.: Dallas, TX, USA, 2005. [Google Scholar]
- Idris, A.C.; Saad, M.R.; Zare-Behtash, H.; Kontis, K. Luminescent measurement systems for the investigation of a scramjet inlet-isolator. Sensors 2014, 14, 6606–6632. [Google Scholar] [CrossRef] [PubMed]
- Lamprecht, B.; Tschepp, A.; Cajlakovic, M.; Sagmeister, M.; Ribitsch, V.; Kostler, S. Luminescence lifetime-based capillary oxygen sensor utilizing monolithically integrated organic photodiodes. Analyst 2013, 138, 5875–5878. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006. [Google Scholar]
- Guerci, P.; Ince, Y.; Heeman, P.; Faber, D.; Ergin, B.; Ince, C. A LED-based phosphorimeter for measurement of microcirculatory oxygen pressure. J. Appl. Phys. 2017, 122, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Kuznetsova, R.T.; Papkovsky, D.B. Solid-state oxygen sensors based on phosphorescent diiodo-borondipyrromethene dye. Sens. Actuators B: Chem. 2015, 212, 229–234. [Google Scholar] [CrossRef]
- Ji, S.; Wu, W.; Wu, Y.; Zhao, T.; Zhou, F.; Yang, Y.; Zhang, X.; Liang, X.; Wu, W.; Chi, L.; Wang, Z.; Zhao, J. Real-time monitoring of luminescent lifetime changes of PtOEP oxygen sensing film with LED/photodiode-based time-domain lifetime device. Analyst 2009, 134, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Borisov, S.; Zenkl, G.; Klimant, I. Phosphorescent platinum(II) and palladium(II) complexes with azatetrabenzoporphyrins - new red laser diode-compatible indicators for optical oxygen sensing. ACS Appl. Mater. Interfaces 2010, 2, 366–374. [Google Scholar] [CrossRef] [PubMed]
- To, W.P.; Liu, Y.; Lau, T.C.; Che, C.M. A robust palladium(II)-porphyrin complex as catalyst for visible light induced oxidative C-H functionalization. Chem. A Eur. J. 2013, 19, 5654–5664. [Google Scholar] [CrossRef] [PubMed]
- Leung, R.W.K.; Yeh, S.C.A.; Fang, Q. Effects of incomplete decay in fluorescence lifetime estimation. Biomed. Opt. Express 2011, 2, 2517–2531. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, P.; Trettnak, W. Effects of polymer matrices on calibration functions of luminescent oxygen sensors based on porphyrin ketone complexes. Anal. Chem. 1996, 68, 2615–2620. [Google Scholar] [CrossRef] [PubMed]
- Palma, A.J.; López-González, J.; Asensio, L.J.; Fernández-Ramos, M.D.; Capitán-Vallvey, L.F. Microcontroller-based portable instrument for stabilised optical oxygen sensor. Sens. Actuators B: Chem. 2007, 121, 629–638. [Google Scholar] [CrossRef]
- Kneas, K.A.; Demas, J.N.; DeGraff, B.A.; Periasamy, A. Fluorescence microscopy study of heterogeneity in polymer-supported luminescence-based oxygen sensors. Microsc. Microanal. 2000, 6, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Payne, S.J.; Fiore, G.L.; Fraser, C.L.; Demas, J.N. Luminescence oxygen sensor based on a ruthenium(II) star polymer complex. Anal. Chem. 2010, 82, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, P.; Leiner, M.J.; Lippitsch, M.E. Response characteristics of luminescent oxygen sensors. Sens. Actuators B: Chem. 1995, 29, 251–257. [Google Scholar] [CrossRef]
- Kelly, C.A.; Toncelli, C.; Kerry, J.P.; Papkovsky, D.B. Discrete O2 sensors produced by a spotting method on polyolefin fabric substrates. Sens. Actuators B: Chem. 2014, 203, 935–940. [Google Scholar] [CrossRef]
- Valeur, B.; Berberan-Santos, M.N. Molecular Fluorescence: Principles and Applications, 2nd ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013. [Google Scholar]
- Koren, K.; Hutter, L.; Enko, B.; Pein, A.; Borisov, S.M.; Klimant, I. Tuning the dynamic range and sensitivity of optical oxygen-sensors by employing differently substituted polystyrene-derivatives. Sens. Actuators B: Chem. 2013, 176, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Borisov, S.M.; Klimant, I. Ultrabright oxygen optodes based on cyclometalated iridium(III) Coumarin Complexes. Anal. Chem. 2007, 79, 7501–7509. [Google Scholar] [CrossRef] [PubMed]
- Carraway, E.R.; Demas, J.N.; DeGraff, B.A. Luminescence quenching mechanism for microheterogeneous systems. Anal. Chem. 1991, 63, 332–336. [Google Scholar] [CrossRef]
- Borisov, S.M.; Nuss, G.; Haas, W.; Saf, R.; Schmuck, M.; Klimant, I. New NIR-emitting complexes of platinum(II) and palladium(II) with fluorinated benzoporphyrins. J. Photochem. Photobiol. A: Chem. 2009, 201, 128–135. [Google Scholar] [CrossRef]
- Woll, D.; Braeken, E.; Deres, A.; De Schryver, F.C.; Uji-i, H.; Hofkens, J. Polymers and single molecule fluorescence spectroscopy, what can we learn? Chem. Soc. Rev. 2009, 38, 313–328. [Google Scholar] [CrossRef] [PubMed]
- Lemon, C.M.; Hwang, S.J.; Maher, A.G.; Powers, D.C.; Nocera, D.G. Halogen photoelimination from SbV dihalide corroles. Inorg. Chem. 2018, 57, 5333–5342. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, P.; Leiner, M.J.P.; Kohlbacher, P. Photobleaching of a ruthenium complex in polymers used for oxygen optodes and its inhibition by singlet oxygen quenchers. Sens. Actuators B: Chem. 1998, 51, 196–202. [Google Scholar] [CrossRef]
- Xue, R.; Behera, P.; Xu, J.; Viapiano, M.S.; Lannutti, J.J. Polydimethylsiloxane core–polycaprolactone shell nanofibers as biocompatible, real-time oxygen sensors. Sens. Actuators B: Chem. 2014, 192, 697–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koren, K.; Borisov, S.M.; Klimant, I. Stable optical oxygen sensing materials based on click-coupling of fluorinated platinum(II) and palladium(II) porphyrins—A convenient way to eliminate dye migration and leaching. Sens. Actuators B: Chem. 2012, 169, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Basu, B.J.; Bharathida, T.; Rikhari, B.; Prasannan, D.; Kum, V.D.; Chakradhar, R.P.S. Studies on the fabrication and characterization of optical sensor coatings for aerodynamic applications. J. Appl. Sci. 2012, 12, 1646–1650. [Google Scholar] [CrossRef]





| Indicator | nm () M cm | nm | ||
|---|---|---|---|---|
| B Band | Q Band | |||
| PdTFPP | 407 (250,000) | 520 (22,800), 553 (20,200) | 673 | 1.0 |
| IrTFPP-CO-Cl | 414 (195,000) | 527 (19,200), 558 (6100) | 681 | 2.3 |
| [O2] (%) | IrTFPP-CO-Cl | ||||
| <> (s) | r | (s) | r | 3 (%) | |
| 0.00 | 102.8 | 0.9999 | 103.1 | 0.9997 | 0.36 |
| 1.01 | 77.19 | 0.9999 | 77.02 | 0.9997 | 0.38 |
| 5.01 | 42.27 | 0.9999 | 39.91 | 0.9995 | 0.57 |
| 9.99 | 27.46 | 0.9999 | 25.62 | 0.9991 | 0.72 |
| 16.00 | 19.91 | 0.9999 | 18.39 | 0.9989 | 0.87 |
| 18.51 | 17.89 | 0.9999 | 16.57 | 0.9988 | 0.94 |
| 21.00 | 16.35 | 0.9999 | 15.09 | 0.9988 | 1.04 |
| [O] (%) | PdTFPP | ||||
| <> (s) | r | (s) | r | 3(%) | |
| 0.00 | 1042 | 0.9999 | 1038 | 0.9999 | 0.27 |
| 1.01 | 327.5 | 0.9999 | 293.4 | 0.9991 | 0.28 |
| 5.01 | 101.2 | 0.9999 | 79.83 | 0.9980 | 0.53 |
| 9.99 | 55.54 | 0.9999 | 42.70 | 0.9975 | 0.66 |
| 16.00 | 36.47 | 0.9998 | 28.52 | 0.9974 | 0.85 |
| 18.51 | 32.55 | 0.9998 | 25.15 | 0.9974 | 1.01 |
| 21.00 | 29.01 | 0.9998 | 22.75 | 0.9972 | 1.04 |
| Indicator | Two-Site Model | Lehrer’s Model | ||||||
|---|---|---|---|---|---|---|---|---|
| <>/<> | f | / | ||||||
| IrTFPP-CO-Cl | 0.9470 | 0.0530 | 0.325 | 0.0189 | 6.3 | 0.9734 | 0.339 | 6.8 |
| PdTFPP | 0.9932 | 0.0068 | 1.994 | 0.0218 | 35.9 | 0.9964 | 2.545 | 45.6 |
| [O] (%) | IrTFPP-CO-Cl | PdTFPP | ||
|---|---|---|---|---|
| (%) | (%) | (%) | (%) | |
| 0.00 | +0.0 | +0.0 | +0.0 | +0.0 |
| 1.01 | +1.9 | +0.6 | +6.5 | +0.1 |
| 5.01 | −0.6 | +0.1 | −0.6 | −1.1 |
| 9.99 | +0.2 | +0.1 | −0.4 | +0.3 |
| 16.00 | −0.1 | −0.1 | +0.7 | −0.2 |
| 18.51 | +0.1 | −0.2 | −0.5 | +0.1 |
| 21.00 | −0.1 | +0.1 | +0.1 | −0.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Moraes Filho, A.F.; Gewehr, P.M.; Maia, J.M.; Jakubiak, D.R. Polystyrene Oxygen Optodes Doped with Ir(III) and Pd(II) meso-Tetrakis(pentafluorophenyl)porphyrin Using an LED-Based High-Sensitivity Phosphorimeter. Sensors 2018, 18, 1953. https://doi.org/10.3390/s18061953
De Moraes Filho AF, Gewehr PM, Maia JM, Jakubiak DR. Polystyrene Oxygen Optodes Doped with Ir(III) and Pd(II) meso-Tetrakis(pentafluorophenyl)porphyrin Using an LED-Based High-Sensitivity Phosphorimeter. Sensors. 2018; 18(6):1953. https://doi.org/10.3390/s18061953
Chicago/Turabian StyleDe Moraes Filho, Alexandre F., Pedro M. Gewehr, Joaquim M. Maia, and Douglas R. Jakubiak. 2018. "Polystyrene Oxygen Optodes Doped with Ir(III) and Pd(II) meso-Tetrakis(pentafluorophenyl)porphyrin Using an LED-Based High-Sensitivity Phosphorimeter" Sensors 18, no. 6: 1953. https://doi.org/10.3390/s18061953





