A Fluorescent Biosensors for Detection Vital Body Fluids’ Agents
Abstract
:1. Introduction
2. Point-Of-Care—Vital Body Fluids’ Agents
3. Fluorescent Biosensors for Determination of Hormones
3.1. Characteristic of the Exemplary Hormones
3.2. Detection Strategies of Working Fluorescent Biosensor for Hormones Monitoring
3.3. Fluorescent Biosensors for Detection of Hormones—Overview
4. Fluorescent Biosensors for Determination of Neurotransmitters
4.1. Characteristic of the Exemplary Neurotransmitters
4.2. Detection Strategies of Working Fluorescent Biosensor for Catecholamines Monitoring
- FRET (Förster Resonance Energy Transfer),
- FLIM (Fluorescence Lifetime Imaging),
- FCS (Fluorescence Correlation Spectroscopy),
- FI (changes in Fluorescence Intensity).
4.3. Fluorescent Biosensors for Detection of Catecholamines—Overview
5. Fluorescent Biosensors for Determination of Key Metabolites
5.1. Characteristic of the Exemplary Key Metabolites
5.2. Detection Strategies of Working Fluorescent Biosensor for Metabolites Monitoring
5.3. Fluorescent Biosensors for Detection of Metabolites—Overview
6. Technology of Microfluidic Systems with Fluorescence Detection
7. LTCC Technology
- 2 blank at the top,
- 4 with 500 µm wide canals and voids for optical fiber and fluidic ports,
- 1 with a 6 × 6 mm2 glass plate and
- 1 at the bottom, with a 2 × 2 mm2 window for fluorescence measurement.
8. Conclusions and Discussion
Funding
Conflicts of Interest
References
- Hall, E.E.H. Biosensors; Prentice-Hall: Englewood Cliffs, NJ, USA, 1991; ISBN 9780130845269. [Google Scholar]
- Mousty, C.; Kaftan, O.; Prevot, V.; Forano, C. Alkaline Phosphatase Biosensors Based on Layered Double Hydroxides Matrices: Role of LDH Composition. Sens. Actuators B Chem. 2008, 133, 442–448. [Google Scholar] [CrossRef]
- Qian, Z.S.; Chai, L.J.; Huang, Y.Y.; Tang, C.; Shen, J.J.; Chen, J.R.; Feng, H. A Real-Time Fluorescent Assay for the Detection of Alkaline Phosphatase Activity Based on Carbon Quantum Dots. Biosens. Bioelectron. 2015, 68, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Zarei, M. Advances in point-of-care technologies for molecular diagnostics. Biosens. Bioelectron. 2017, 98, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Schabowski, J.; Nicer, T.; Mardarowicz, M. “Przyłóżkowe” szybkie testy laboratoryjne–przydatność w podstawowej opiece zdrowotnej. Forum Medycyny Rodzinnej 2008, 2, 358–364. [Google Scholar]
- Drain, D.K.; Hyle, E.P.; Naubary, F.; Freedberg, K.A.; Wilson, D.; Bishai, W.R. Diagnostic point-of-care tests in resource-limited settings. Lancet Infect. Dis. 2014, 14, 239–249. [Google Scholar] [CrossRef] [Green Version]
- Eswald, M.; Fechner, P.; Gauglitz, G. A multi-analyte biosensor for the simultaneous label-free detection of pathogens and biomarkers in point-of-care animal testing. Anal. Bioanal. Chem. 2015, 407, 4005–4013. [Google Scholar] [CrossRef] [PubMed]
- Nowak, L.; Nauzil, P.; Pipper, J.; Zhang, Y.; Lee, S. An integrated fluorescence detection system for lab-on-a-chip applications. Lab Chip 2007, 7, 27–29. [Google Scholar] [CrossRef]
- Webster, J.R.; Burns, M.A.; Burke, D.T.; Mastrangelo, C.H. Monolithic capillary electrophoresis device with integrated fluorescence detector. Anal. Chem. 2001, 73, 1622–1626. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.L.; Fang, Q.; Zhang, T.; Jin, X.H.; Fang, Z.L. Laser-induced fluorescence detection system for microfluidic chips based on an orthogonal optical arrangement. Anal. Chem. 2006, 78, 3827–3834. [Google Scholar] [CrossRef] [PubMed]
- Kamei, T.; Wada, T. Contact-lens type of micromachined hydrogenated amorphous Si fluorescence detector coupled with microfluidic electrophoresis devices. Appl. Phys. Lett. 2006, 89, 114101. [Google Scholar] [CrossRef]
- Klotz, A.; Brecht, A.; Barzen, C.; Gauglitz, G.; Harris, R.D.; Quigley, G.R.; Wilkinson, J.S.; Abuknesha, R.A. Waveguide immunofluorescence sensor for water analysis. Sens. Actuators B Chem. 1998, 51, 181–187. [Google Scholar] [CrossRef]
- Piunno, P.A.; Barzda, V.; Jantzi, S.C.; Kotoris, C.C.; Major, A.; Musikhin, S.; Raha, S.; Krull, U.J. Toward the development of optical nucleic acid biosensors based on TIRF and TCSPC for high sensitivity determinations. Proc. SPIE 2005, 5969, 59690R. [Google Scholar] [CrossRef]
- Barrettoni, C.; Berneschi, S.; Bernini, R.; Giannetti, A.; Grimaldi, I.A.; Persichetti, G.; Testa, G.; Tombelli, S.; Trono, C.; Baldini, F. Optical monitoring of therapeutic drugs with a novel fluorescence-based POCT device. Procedia Eng. 2014, 87, 392–395. [Google Scholar] [CrossRef]
- Seidel, M.; Niessner, R. Automated analytical microarrays: A critical review. Anal. Bioanal. Chem. 2008, 391, 1521–1544. [Google Scholar] [CrossRef] [PubMed]
- Gauglitz, G.G. Optical sensing: Recognition elements and devices. Proc. SPIE 2012, 8545, 85450F. [Google Scholar] [CrossRef]
- Tschmelak, J.; Proll, G.; Gauglitz, G. Verification of performance with the automated direct optical TIRF immunosensor (river analyser) in single and multi-analyte assays with real water samples. Biosens. Biolelectron. 2004, 20, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Gauglitz, G.; Proll, G. Total internal reflection fluorescence sensing-quality assurance, application to water analysis. Standar. Qual. Assur. Fluoresc. Meas. 2008, 5, 415–428. [Google Scholar] [CrossRef]
- Gauglitz, G. Direct optical detection in bioanalysis: An update. Anal. Bioanal. Chem. 2010, 398, 2363–2372. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.F.; Fu, C.; Chen, Y.T.; Jou, A.F.J.; Chen, C.C.; Chou, C.; Annie, H.J. Use of liposomal amplifiers in total internal reflection fluorescence fiber-optic biosensors for protein detection. Biosens. Bioelectron. 2016, 77, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Berner, M.; Hilbig, U.; Schubert, M.B.; Gauglitz, G. Laser-induced fluorescence detection platform for point-of-care testing. Meas. Sci. Technol. 2017, 28, 85701. [Google Scholar] [CrossRef]
- Luka, G.; Ahmadi, A.; Hoorfar, M. Microfluidics Integrated biosensors: A Leading Technology towards Lab-on-a-Chip and Sensing Applications. Sensors 2015, 15, 30011–30031. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Chakma, B.; Patra, S.; Goswami, P. Potential biomarkers and their applications for rapid and reliable detection of malaria. BioMed Res. Int. 2014, 2014, 852645. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Chen, S.; Huang, W.; Fang, G.; Hua, P.; Wang, S. Study on an on-line molecularly imprinted solid-phase extraction coupled to high performance liquid chromatography for separation and determination of trace estrone in environment. Anal. Bioanal. Chem. 2009, 393, 1273–1279. [Google Scholar] [CrossRef] [PubMed]
- Segner, H.; Caroll, K.; Fenske, M.; Janssen, C.R.; Maack, G.; Pascoe, D.; Schafers, C.; Vandenbergh, G.F.; Watts, M.; Wenzel, A. Identification of endocrine-disrupting effects in aquatic vertebrates and invertebrates: Report from the European IDEA project. Ecotoxicol. Environ. Saf. 2003, 54, 302–314. [Google Scholar] [CrossRef]
- Mills, L.; Chichester, C. Review of evidence: Are endocrine-disrupting chemicals in the aquatic environment impacting fish populations? Sci. Total Environ. 2005, 343, 1–292. [Google Scholar] [CrossRef] [PubMed]
- Penalyer, A.; Pocurull, E.; Borrull, F.; Marce, R.M. Method based on solid phase microextraction—High performance liquid chromatography with UV and electrochemical detection to determine estrogenic compounds in water samples. J. Chromatogr. A 2002, 964, 153–160. [Google Scholar] [CrossRef]
- Houtman, C.; Sterk, S.; van de Heijning, M.; Brouwer, A.; Stephany, R.; van der Burg, B.; Sonneveld, E. Detection of anabolic androgenic steroid abuse in doping control using mammalian reporter gene bioassays. Anal. Chim. Acta 2009, 637, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Botr, F. Drugs of abuse and abuse of drugs in sportsmen: The role of in vitro models to study effects and mechanisms. Toxicol. In Vitro 2003, 17, 509–513. [Google Scholar] [CrossRef]
- Beato, M.; Herrlich, P.; Schutz, G. Steroid hormone receptors: Many Actors in search of a plot. Cell 1995, 8, 851–857. [Google Scholar] [CrossRef]
- Bahadir, E.; Sezgintürk, M. Electrochemical biosensors for hormone analysis. Biosens. Bioelectron. 2015, 68, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Arvinte, A.; Westermann, C.; Sesay, A.; Virtanen, V. Electrocatalytic oxidation and determination of insulin at CNT-nickel-cobalt oxide modified electrode. Sens. Actuators B Chem. 2010, 150, 756–763. [Google Scholar] [CrossRef]
- Schirhagl, R.; Latif, U.; Podlipna, D.; Blumenstock, H.; Dickert, F.L. Natural and Biomimetic Materials for the detection of Insulin. Anal. Chem. 2012, 84, 3908–3913. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zuo, F.; Tan, X.; Xu, S.; Yuan, R.; Chen, S. A novel electrochemiluminescent biosensor based on resonance energy transfer between poly(9,9-di-N-octylfluorenyl-2,7-diyl) and 3,4,9,10-perylenetetracar-boxylic acid for insulin detection. Biosens. Bioelectron. 2018, 104, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Pires, N.; Dong, T.; Hanke, U.; Hoivik, N. Recent Developments in Optical Detection Technologies in Lab-on-a-Chip Devices for Biosensig Applications. Sensors 2014, 14, 15458–15479. [Google Scholar] [CrossRef] [PubMed]
- Ming, W.; Wang, X.; Lu, W.; Yhang, Y.; Song, X.; Li, J.; Chen, L. Magnetic molecularly imprinted polymers for the fluorescent detection of trace 17β-estradiol in environmental water. Sens. Actuators B Chem. 2017, 238, 1309–1315. [Google Scholar] [CrossRef]
- Yildirim, N.; Long, F.; Gao, C.; He, M.; Shi, H.; Gu, A.Z. Aptamer-based Optical Biosensor For Rapid and Sensitive Detection of 17β-estradiol In Water Samples. Environ. Sci. Technol. 2012, 46, 3288–3294. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Li, W.; Zhang, Y.; Zhang, C.; Liu, G.; Wang, S. An ultrasensitive detection of 17β-estradiol using a gold nanoparticles-based fluorescence immunoassay. Analyst 2015, 140, 2001–2007. [Google Scholar] [CrossRef] [PubMed]
- Tschmelak, J.; Kumpf, M.; Kappel, N.; Proll, G.; Gauglitz, G. Total internal reflectance fluorescence (TIRF) biosensor for environmental monitoring of testosterone with commercially available immunochemistry: Antibody characterization, assay development and real sample measurement. Talanta 2006, 69, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, D.; Zhang, P.; Gong, P.; Chen, C.; Gao, G.; Cai, L. A near infrared fluorescence resonance energy transfer based aptamer biosensor for insulin detection in human plasma. Chem. Commun. 2014, 50, 811–813. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, H.S.; Sao, R.; Vaish, R. Label free selective detection of estriol using graphene oxide-based fluorescence sensor. J. Appl. Phys. 2014, 116, 034701. [Google Scholar] [CrossRef]
- Allen, S.L. Regulatory Aspects of Acute Neurotoxicity Assessment. In Hayes’ Handbook of Pesticide Toxicology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2010; pp. 587–602. [Google Scholar]
- Perry, M.; Li, Q.; Kennedy, R.T. Review of recent advances in analytical techniques for the determination of neurotransmitters. Anal. Chim. Acta 2009, 653, 1–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schermann, J.-P. Spectroscopy and Modeling of Biomolecular Building Blocks, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 2008; pp. 309–321. ISBN 978-0-444-52708-0. [Google Scholar]
- Merims, D.; Giladi, N. Dopamine dysregulation syndrome, addiction and behavioral changes in Parkinson’s disease. Parkinsonism Relat. Disord. 2008, 14, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Peskind, E.R.; Elrod, R.; Dobie, D.J.; Pascualy, M.; Petrie, E.; Jensen, C.; Brodkin, K.; Murra, S.; Veith, R.C.; Raskind, M.A. Cerebrospinal Fluid Epinephrine in Alzheimer’s Disease and Normal Aging. Neuropsychopharmacology 1998, 19, 465–471. [Google Scholar] [CrossRef]
- Tao, Y.; Lin, Y.; Ben, J.; Qu, X. A dual fluorimetric and colorimetric sensor for dopamine based on BSA-stabilized Au-nanoclusters. Biosens. Bioelectron. 2013, 42, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Gunduz-Bruce, H. The acute effects of NMDA antagonism: From the rodent to the human brain. Brain Res. Rev. 2009, 60, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, B.W.; Nemeroff, C.B. The Role of Dopamine in the Pathophysiology of Depression. Arch. Gen. Psychiatry 2007, 64, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Stroumpoulis, K.; Xanthos, T.; Rokas, G.; Kitsou, V.; Papadimitriou, D.; Serpetinis, I.; Perrea, D.; Papadimitriou, L.; Kouskouni, E. Vasopressin and epinephrine in the treatment of cardiac arrest: An experimental study. Crit. Care 2008, 12, R40. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.; Debs, L.H.; Patel, A.P.; Nguyen, D.; Patel, K.; O’Connor, G.; Grati, M.; Mittal, J.; Yan, D.; Eshraghi, A.A.; et al. Neurotransmitters: The Critical Modulators Regulating Gut-Brain Axis. J. Cell. Physiol. 2017, 232, 2359–2372. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.A.; Arundell, M.; Parker, K.H.; Yeoman, M.S.; O’Hare, D. Simple and rapid determination of serotonin and catecholamines in biological tissue using high performance liquid chromatography with electrochemical detection. J. Chromatogr. B 2005, 818, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Fotopoulou, M.A.; Ioannou, P.C. Post-column terbium complexation and sensitized fluorescence detection for the determination of norepinephrine, epinephrine and dopamine using high-performance liquid chromatography. Anal. Chim. Acta 2002, 462, 179–185. [Google Scholar] [CrossRef]
- Zhang, L.; Qv, S.; Wang, Z.; Cheng, J. Determination of dopamine in single rat pheochromocytoma cell by capillary electrophoresis with amperometric detection. J. Chromatogr. B 2003, 792, 381–385. [Google Scholar] [CrossRef]
- Heien, M.L.A.V.; Johnson, M.A.; Wightman, R.M. Resolving Neurotransmitters Detected by Fast-Scan Cyclic Voltammetry. Anal. Chem. 2004, 76, 5697–5704. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ding, L.; Wang, Q.; Su, B. Differential pulse voltammetry detection of dopamine and ascorbic acid by permselective silica mesochannels vertically attached to the electrode surface. Analyst 2014, 139, 3926–3931. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, M.; Naccarato, A.; Sindona, G.; Tagarelli, A. A reliable and simple method for the assay of neuroendocrine tumor markers in human urine by solid-phase microextraction–gas chromatography-triple quadrupole mass spectrometry. Anal. Chim. Acta 2013, 759, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Nagaraja, P.; Vasantha, R.A.; Sunitha, K.R. A sensitive and selective spectrophotometric estimation of catechol derivatives in pharmaceutical preparations. Talanta 2001, 55, 1039–1046. [Google Scholar] [CrossRef]
- Eisenhofer, G.; Kopin, I.J.; Goldstein, D.S. Catecholamine Metabolism: A Contemporary View with Implications for Physiology and Medicine. Pharmacol. Rev. 2004, 56, 331–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patrick, G.L. An Introduction to Medicinal Chemistry, 4th ed.; Oxford University Press: Oxford, UK, 2009; pp. 611–612. ISBN 978-0-19-107391-5. [Google Scholar]
- Marc, D.T.; Ailts, J.W.; Campeau, D.C.A.; Bull, M.J.; Olson, K.L. Neurotransmitters excreted in the urine as biomarkers of nervous system activity: Validity and clinical applicability. Biobehav. Rev. 2011, 35, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Tolosa, E.; Jankovic, J.J. Parkinson’s Disease and Movement Disorders, 5th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; pp. 271–283. ISBN 978-0-7817-7881-7. [Google Scholar]
- Ota, M.; Yasuno, F.; Ito, H.; Seki, C.; Nozaki, S.; Asada, T.; Suhara, T. Age-related decline of dopamine synthesis in the living human brain measured by positron emission tomography with L-[β-11C]DOPA. Life Sci. 2006, 79, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Moghaddam, A.B.; Hosseini, S.; Kazemzad, M.; Dinarvand, R. A norephinephrine biosensor based on a glassy carbon electrode modified with carbon nanotubes. Anal. Methods 2011, 3, 2406–2411. [Google Scholar] [CrossRef]
- Adran, S.; Stanley, S.; Wong, K.; Otiniano, E.; Amighi, A.; Baudry, M. Fluorescence resonance energy transfer (FRET)-based biosensors: Visualizing cellular dynamics and bioenergetics. Appl. Microbiol. Biotechnol. 2012, 96, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Kłos-Witkowska, A. Biosensors and fluorescent sensors. Meas. Autom. Monit. 2014, 60, 3. [Google Scholar]
- Ochi, R.; Pavone, F. Non-linear fluorescence lifetime imaging of biological tissues. Anal. Bioanal. Chem. 2011, 400, 2687–2697. [Google Scholar] [CrossRef]
- Urnworth, M.; Rowan, S.; Weder, C.H. Fluorescent sensors for detection of chemical walfare agents. Chem. Eur. J. 2017, 13, 7828–7836. [Google Scholar] [CrossRef] [PubMed]
- D’Auria, S.; Lakowicz, J. Enzyme fluorescence as a sensing tool: New perspectives in biotechnology. Curr. Opin. Biotechnol. 2001, 12, 99–104. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, C.; Wang, C.; Pu, F.; Ren, J.; Qu, X. Silver nanoprobe for sensitive and selective colorimetric detection of dopaminevia robust Ag–catechol interaction. Chem. Commun. 2011, 47, 1181–1183. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Kim, J. Fabrication of a Dopamine Sensor Based on Carboxyl Quantum Dots. J. Nanosci. Nanotechnol. 2015, 15, 7871–7875. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.I.B.; Ferreira, F.D.P.; Freitas, A.C.; Rocha-Santos, T.A.P.; Durate, A.C. Optical fiber biosensor coupled to chromatographic separation for screening of dopamine, norepinephrine and epinephrine in human urine and plasma. Talanta 2009, 80, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.P.; Wang, S.Q.; Lin, X.Q. Fabrication of layer-by-layer deposited multilayer films containing DNA and gold nanoparticle for norepinephrine biosensor. Anal. Chim. Acta 2004, 519, 161–166. [Google Scholar] [CrossRef]
- Mohsin, M.A.; Liu, B.D.; Zhang, X.L.; Yang, W.J.; Liu, L.S.; Jiang, X. Cellular-memblutarane inspired surface modification of well aligned ZnO nanorods for chemosensing of epinephrine. RSC Adv. 2017, 7, 3012–3020. [Google Scholar] [CrossRef]
- Baron, R.; Zayats, M.; Willner, I. Dopamine-, L-DOPA-, Adrenaline-, and Noradrenaline-Induced Growth of Au Nanoparticles: Assays for the Detection of Neurotransmitters and of Tyrosinase Activity. Anal. Chem. 2005, 77, 1566–1571. [Google Scholar] [CrossRef] [PubMed]
- Nikolelis, D.P.; Drivelos, D.A.; Simantirali, M.G.; Koinis, S. An Optical Spot Test for the Detection of Dopamine in Human Urine Using Stabilized in Air Lipid Films. Anal. Chem. 2004, 76, 2174–2180. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yan, X.; Meng, K.; Wang, S. Graphene Oxide Based Photoinduced Charge Transfer Label-Free Near-Infrared Fluorescent Biosensor for Dopamine. Anal. Chem. 2011, 83, 8787–8793. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Yin, M.; Pu, F.; Ren, J.; Qu, X. DNA-Templated Silver Nanoparticles as a Platform for Highly Sensitive and Selective Fluorescence Turn-On Detection of Dopamine. Small 2011, 7, 1557–1561. [Google Scholar] [CrossRef] [PubMed]
- Aswathy, B.; Sony, G. Cu2+ modulated BSA–Au nanoclusters: A versatile fluorescence turn-on sensor for dopamine. Microchem. J. 2014, 116, 151–156. [Google Scholar] [CrossRef]
- Baluta, S.; Malecha, K.; Zając, D.; Sołoducho, J.; Cabaj, J. Dopamine sensing with fluorescence strategy based on low temperature co-fired ceramic technology modified with conducting polymers. Sens. Actuators B Chem. 2017, 252, 803–812. [Google Scholar] [CrossRef]
- Weng, S.; Liang, D.; Qiu, H.; Liu, Z.; Lin, Z.; Zheng, Z.; Liu, A.; Chen, W.; Lin, X. A unique turn-off fluorescent strategy for sensing dopamine based on formed polydopamine (pDA) using graphene quantum dots (GQDs) as fluorescent probe. Sens. Actuators B Chem. 2015, 221, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Shi, F.; Zhao, X.; Chen, L.; Su, X. 3-Aminophenyl boronic acid-functionalized CuInS2 quantum dots as a near-infrared fluorescence probe for the determination of dopamine. Biosens. Bioelectron. 2013, 47, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Huang, Y.; Guo, T.; Sun, L.; Guan, B. Mesoporous nanospheres functionalized optical microfiber biosensor for low concentration neurotransmitter detection. Opt. Express 2016, 24, 27152–27159. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Shan, L.; Yue, J.; Wang, X. Spectrophotometric determination of total serotonin derivatives in the safflower seeds with Ehrlich’s reagent and the underlying color reaction mechanism. Food Chem. 2008, 108, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Xu, H.; Li, Y.; Ma, S.; Zhong, X. Adenosine capped QDs based fluorescent sensor for detection of dopamine with high selectivity and sensitivity. Analyst 2014, 139, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Rassaei, L.; Olthuis, W.; Tsujimura, S.; Sudhölter, E.J.R.; van der Berg, A. Lactate biosensors: Current status and outlook. Anal. Bianal. Chem. 2014, 406, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Kemp, G. Lactate accumulation, proton buffering, and pH change in ischemically excersing muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 298, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A. Anaerobic threshold: Review of the concept and direction for future research. Med. Sci. Sports Excerc. 1985, 17, 22–34. [Google Scholar] [CrossRef]
- Guan, W.; Duan, X.; Reed, M.A. Highly specific and sensitive non-enzymatic determination of uric acid in serum and urine by extended gate field effect transistor sensors. Biosens. Bioelectron. 2013, 51, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, I.; Chudy, M.; Dybko, A.; Brzózka, Z. Uric acid determination in a miniaturized flow system with dual optical detection. Sens. Actuators B Chem. 2008, 130, 508–513. [Google Scholar] [CrossRef]
- Azmi, N.E.; Ramli, N.I.; Abdullah, J.; Abdul Hamid, M.A.; Sidek, H.; Abd Rahman, S.; Ariffin, N.; Yusof, N.A. A simple and sensitive fluorescence based biosensor for determination of uric acid using H2O2-sensitive quantum dots/dual enzymes. Biosens. Bioelectron. 2015, 67, 129–133. [Google Scholar] [CrossRef] [PubMed]
- He, J.B.; Jin, G.P.; Chen, Q.Z.; Wang, Y. A quercetin-modified biosensor for amperometric determination of uric acid in the presence of ascorbic acid. Anal. Chim. Acta 2007, 585, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, H.; Hou, T. Simultaneous determination of dopamine, ascorbic acid and uric acid with electrospun carbon nanofibers modified electrode. Electrochem. Commun. 2008, 10, 1431–1434. [Google Scholar] [CrossRef]
- Kumar, A.; Hens, A.; Arun, R.K.; Chatterje, M.; Mahato, K.; Layek, K.; Chanda, N. A paper based microfluidic device for easy detection of uric acid using positively charged gold nanoparticles. Analyst 2015, 140, 1817–1821. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.Q.; Huang, N.; Lu, Q.J.; Liu, M.I.; Li, H.T.; Zhang, Y.Y.; Yao, S.Z. A quadruplet electrochemical platform for ultrasensitive and simultaneous detection of ascorbic acid, dopamine, uric acid and acetaminophen based on a ferrocene derivative functional Au NPs/carbon dots nanocomposite and graphene. Anal. Chim. Acta 2016, 903, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Li, G.; Jiang, Y.Z.; Kang, D.Z.; Jin, C.H.; Shi, Q.; Jin, T.F.; Inoue, K.; Todoroki, K.; Toyo’oka, T.; et al. Human nails metabolite analysis: A rapid and simple method for quantification of uric acid in human fingernail by high-performance liquid chromatography with UV-detection. J. Chromatgr. B 2015, 1002, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Cui, P.; Chen, X.X.; Ye, Q.Q.; Ye, M.G.; Li, L.; Wang, A. DNA, hybridization detection based on fluorescence resonance energy transfer between dye-doped core-shell silica nanoparticles and gold nanoparticles. Analyst 2011, 136, 3973–3980. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, B.D.; Chaubey, A. Biosensors for clinical diagnostics industry. Sens. Actuators B Chem. 2003, 91, 117–127. [Google Scholar] [CrossRef]
- Moschou, E.A.; Sharma, B.V.; Deo, S.K.; Daunert, S. Fluorescence Glucose Detection: Advances toward the Ideal In Vivo Biosensor. J. Fluoresc. 2004, 14, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Sierra, J.F.; Galban, J.; Castillo, J.R. Determination of glucose in blood based on the intrinsic fluorescence of glucose oxidase. Anal. Chem. 1997, 69, 1471–1476. [Google Scholar] [CrossRef]
- Long, Q.; Fang, A.; Wen, Y.; Li, H.; Zhang, Y.; Yao, S. Rapid and highly-sensitive uric acid sensing based on enzymatic catalysis-induced upconversion inner filter effect. Biosens. Bioelectron. 2016, 86, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-S.; Ju, X.; Ga, X.-F.; Ya, W. A Novel Immobilization Enzyme Lactated Fluorescence Capillary Biosensor. Chin. J. Anal. Chem. 2009, 37, 637–642. [Google Scholar] [CrossRef]
- Zheng, X.T.; Yang, H.B.; Li, C.M. Optical Detection of Single Cell Lactate Release for Cancer Metabolic Analysis. Anal. Chem. 2010, 82, 5082–5087. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Qin, Y.; Huang, Y.; Yang, R.; Hou, L.; Ye, F.; Zhao, S. A label-free fluorescence assay for hydrogen peroxide and glucose based on the bifunctional MIL-53(Fe) nanozyme. Chem. Commun. 2018, 54, 1762–1765. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tse, W.H.; Chen, Y.; McDonald, M.W.; Melling, J.; Zhang, J. Nanostructured biosensor for detecting glucose in tear by applying fluorescence resonance energy transfer quenching mechanism. Biosens. Bioelectron. 2017, 91, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Ayranci, R.; Kirbay, F.O.; Demirkol, D.O.; Ak, M.; Timur, S. Copolymer based multifunctional conducting polymer film for fluorescence sensing of glucose. Methods Appl. Fluoresc. 2018, 6, 035012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Augustyniak, I.; Kubicki, W.; Dziuban, J.; Lizanets, D.; Matviykiv, O.; Lobur, M. Measurement set-up for lab-on-a-chip fluorimetric detection. In Proceedings of the 21st International Conference Mixed Design of Integrated Circuits and Systems (MIXDES), Lublin, Poland, 19–21 June 2014; IEEE: Piscataway, NJ, USA, 2014. [Google Scholar] [CrossRef]
- Walczak, R.; Kubicki, W.; Dziuban, J. Low cost fluorescence detection using a CCD array and image processing for on-chip gel electrophoresis. Sens. Actuators B Chem. 2017, 240, 46–54. [Google Scholar] [CrossRef]
- Guo, L.; Shi, Y.; Liu, X.; Han, Z.; Zhao, Z.; Chen, Y.; Xie, W.; Li, X. Enhanced fluorescence detection of proteins using ZnO nanowires integrated inside microfluidic chips. Biosens. Bioelectron. 2018, 99, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.M.; Chang, C.C. The portable fluorescence detection system matched with PDMS microfluidic biochip for DNA hybridization detection. Optik 2015, 126, 2600–2605. [Google Scholar] [CrossRef]
- Waheed, S.; Cabot, J.M.; Macdonald, N.P.; Lewis, T.; Guijt, R.M.; Paull, B.; Breadmore, M.C. 3D printed microfluidic devices: Enablers and barriers. Lab Chip 2016, 16, 1993–2013. [Google Scholar] [CrossRef] [PubMed]
- Adamski, K.; Kubicki, W.; Walczak, R. 3D Printed Electrophoretic Lab-on-chip for DNA Separation. Procedia Eng. 2016, 168, 1454–1457. [Google Scholar] [CrossRef]
- Kadimisetty, K.; Song, J.; Doto, A.M.; Hwang, Y.; Peng, J.; Mauk, M.G.; Bushman, F.D.; Gross, R.; Jarvis, J.N.; Liu, C. Fully 3D printed integrated reactor array for point-of-care molecular diagnostics. Biosens. Bioelectron. 2018, 109, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Golonka, L.J.; Roguszczak, H.; Zawada, T.; Radojewski, J.; Grabowska, I.; Chudy, M.; Dybko, A.; Brzózka, Z.; Stadnik, D. LTCC based microfluidic system with optical detection. Sens. Actuators B Chem. 2005, 111–112, 396–402. [Google Scholar] [CrossRef]
- Bembnowicz, P.; Małodobra, M.; Kubicki, W.; Szczepańska, P.; Górecka-Drzazga, A.; Dziuban, J.; Jonkisz, A.; Karpiewska, A.; Dobosz, T.; Golonka, L.J. Preliminary studies on LTCC based PCR microreactor. Sens. Actuators B Chem. 2010, 150, 715–721. [Google Scholar] [CrossRef]
- Bembnowicz, P.; Golonka, L.J. Integration of transparent glass window with LTCC technology for μTAS application. J. Eur. Ceram. Soc. 2010, 30, 743–749. [Google Scholar] [CrossRef]
- Malecha, K.; Czok, M.; Hetnar, A.; Pawlik, A.; Sztajer, H.; Golonka, L.J. Micro Ceramic Cell Analyzer (MCCA)—Preliminary results. Microelectron. Reliab. 2011, 51, 1250–1252. [Google Scholar] [CrossRef]
- Czok, M.; Tadaszak, R.; Bembnowicz, P.; Golonka, L.J. LTCC based chip for monitoring in biological applications. Sens. Actuators B Chem. 2013, 189, 118–122. [Google Scholar] [CrossRef]
- Malecha, K. A PDMS–LTCC bonding using atmospheric pressure plasma for microsystem applications. Sens. Actuators B Chem. 2013, 181, 486–493. [Google Scholar] [CrossRef]
- Malecha, K. The utilization of LTCC-PDMS bonding technology for microfluidic system applications—A simple fluorescent sensor. Microelectron. Int. 2016, 33, 149–154. [Google Scholar] [CrossRef]
- Couceiro, P.; Gomez-de Pedro, S.; Alonso-Chamarro, J. All-ceramic analytical microsystems with monolithically integrated optical detection microflow cells. Microfluid. Nanofluid. 2015, 18, 649–656. [Google Scholar] [CrossRef]
- Couceiro, P.; Alonso-Chamarro, J. Microfabrication of Monolithic Microfluidic Platforms Using Low Temperature Co-Fired Ceramics Suitable for Fluorescence Imaging. Anal. Chem. 2017, 89, 9147–9153. [Google Scholar] [CrossRef] [PubMed]
- Malecha, K. The Implementation of Fluorescence-Based Detection in LTCC (Low-Temperature-Co-Fired-Ceramics) Microfluidic Modules. Int. J. Appl. Ceram. Technol. 2016, 13, 69–77. [Google Scholar] [CrossRef]
- Golonka, L.J. Technology and applications of Low Temperature Cofired Ceramic (LTCC) based sensors and microsystems. Bull. Pol. Acad. Sci. Tech. Sci. 2006, 54, 221–231. [Google Scholar]
- Gongora-Rubio, M.R.; Espinoza-Vallejos, P.; Sola-Laguna, L.; Santiago-Aviles, J.J. Overview of low temperature co-fired ceramics tape technology for meso-system technology (MsST). Sens. Actuators A Phys. 2001, 89, 222–241. [Google Scholar] [CrossRef]
- Vashist, S.K.; Luong, J.H. Trends in in vitro diagnostics and mobile healthcare. Biotechnol. Adv. 2016, 34, 137–138. [Google Scholar] [CrossRef] [PubMed]
- Vashist, S.K.; Luppa, P.B.; Yeo, L.Y.; Ozcan, A.; Luong, J.H. Emerging Technologies for Next-Generation Point-of-Care Testing. Trends Biotechnol. 2015, 33, 692–705. [Google Scholar] [CrossRef] [PubMed]
- Vashist, S.K.; Mudanyali, O.; Schneider, E.M.; Zengerle, R.; Ozcan, A. Cellphone-based devices for bioanalytical sciences. Anal. Bioanal. Chem. 2014, 406, 3263–3277. [Google Scholar] [CrossRef] [PubMed]
- Vashist, S.K.; Schneider, E.M.; Luong, J.H. Commercial Smartphone-Based Devices and Smart Applications for Personalized Healthcare Monitoring and Management. Diagnostics 2014, 4, 104–128. [Google Scholar] [CrossRef] [PubMed]
- Yager, P.; Edwards, T.; Fu, E.; Helton, K.; Nelson, K.; Tam, M.R.; Weigl, B.H. Microfluidic diagnostic technologies for global public health. Nature 2006, 442, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Pollock, N.R.; Rolland, J.P.; Kumar, S.; Beattie, P.D.; Jain, S.; Noubary, F.; Wong, V.L.; Pohlmann, R.A.; Ryan, U.S.; Whitesides, G.M. A paper-based multiplexed transaminase test for low-cost, point-of-care liver function testing. Sci. Transl. Med. 2012, 4, 152ra129. [Google Scholar] [CrossRef] [PubMed]
- Hugo, S.; Land, K.; Madou, M.; Kido, H. A centrifugal microfluidic platform for point-of-care diagnostic applications. S. Afr. J. Sci. 2014, 110, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Jing, W.; Jiang, X.; Zhao, W.; Liu, S.; Cheng, X.; Sui, G. Microfluidic Platform for Direct Capture and Analysis of Airborne Mycobacterium tuberculosis. Anal. Chem. 2014, 86, 5815–5821. [Google Scholar] [CrossRef] [PubMed]





| Biomarker | Sensing Platform | Transduction Type | LOD | References |
|---|---|---|---|---|
| 17β-estradiol | Aptamers specific for 17β-estradiol | Evanescent Wave | 2.1 nM | [37] |
| 17β-estradiol | anti-E2 antibody | Change in fluorescence intensity | 6.37 × 10−6 ng mL−1 | [38] |
| 17β-estradiol | Fluorescein | Change in fluorescence intensity | 30 nM | [36] |
| Estriol | Graphene oxide/estriol complex | FRET | 1.3 nM | [41] |
| Testosterone | Anti-testosterone antibody | TIRF | 0.2 ng L−1 | [39] |
| Insulin | Aptamers specific for insulin | FRET | 0.72 pM | [40] |
| Insulin | Anti-insulin antibody | FRET | 3.0 × 10−6 ng/mol | [34] |
| Biomarker | Sensing Platform | Transduction Type | LOD | References |
|---|---|---|---|---|
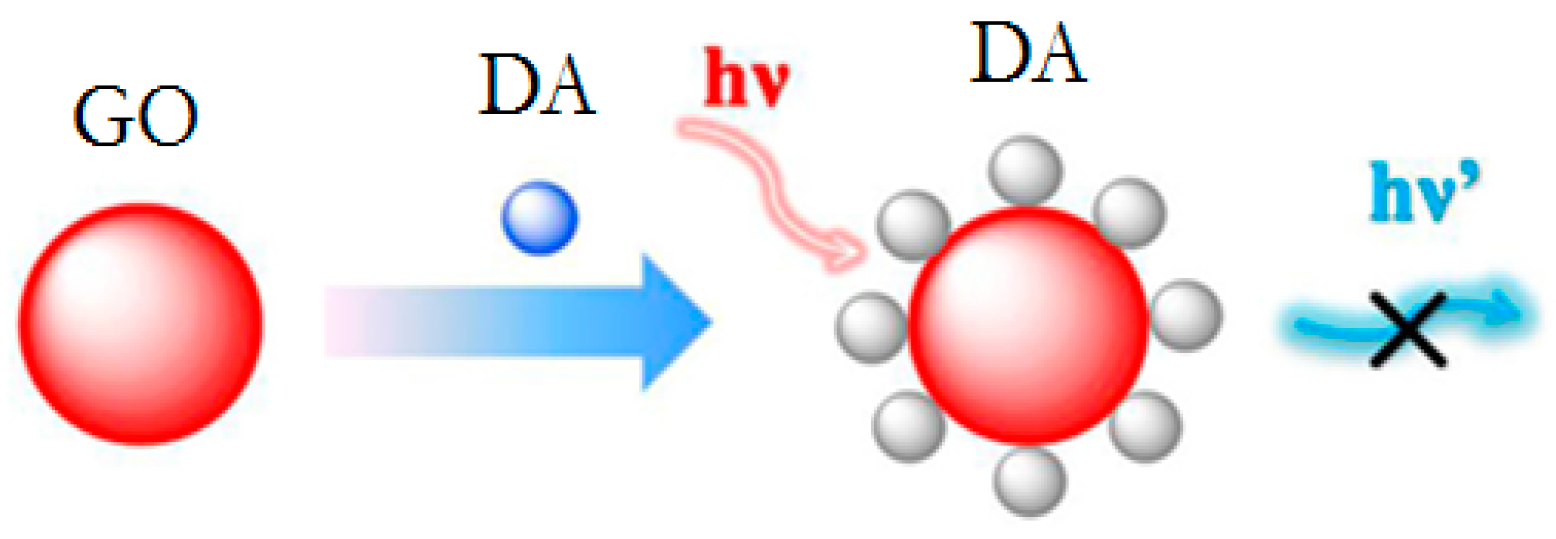
| DA | GQDs/pDA complex | FRET | 8 nM | [81] |
| DA | Functionalized-CuInS2 QDs | FI | 200 nM | [82] |
| 5HT | APTES-functionalized surface-assembly of Ag@mSiO2 | Interferometry | 84 fM | [83] |
| 5HT | Ehrlich’s reagent-5HT complex | Spectrophotometry | 2.3 μM | [84] |
| DA | dithienotetraphenylsilane/laccase GQDs/pDA complex | FRET | 80 nM | [80] |
| DA | CdSe/ZnS QDs/A | FI | 29.3 nM | [85] |
| Biomarker | Sensing Platform | Transduction Type | LOD | References |
|---|---|---|---|---|
| Lactate | LDH/medical capillary | Change in fluorescence intensity | 0.45 mM | [102] |
| Lactate | LDH/optical fiber | Change in fluorescence intensity | 20 μM | [103] |
| Uric acid | QDs/uricase/HRP | FRET | 125 μM | [91] |
| Uric acid | UCNPs/uricase | IFE | 6.7 μM | [101] |
| Glucose | MIL-53(Fe)/GOx | Change in fluorescence intensity | 8.44 × 10−9 mol L−1 | [104] |
| Glucose | CdSe/ZnS QDs/concanavalin A | FRET | - | [105] |
| Glucose | RDC/SNS/GOx | Change in fluorescence intensity | 1.2 nM | [106] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nawrot, W.; Drzozga, K.; Baluta, S.; Cabaj, J.; Malecha, K. A Fluorescent Biosensors for Detection Vital Body Fluids’ Agents. Sensors 2018, 18, 2357. https://doi.org/10.3390/s18082357
Nawrot W, Drzozga K, Baluta S, Cabaj J, Malecha K. A Fluorescent Biosensors for Detection Vital Body Fluids’ Agents. Sensors. 2018; 18(8):2357. https://doi.org/10.3390/s18082357
Chicago/Turabian StyleNawrot, Witold, Kamila Drzozga, Sylwia Baluta, Joanna Cabaj, and Karol Malecha. 2018. "A Fluorescent Biosensors for Detection Vital Body Fluids’ Agents" Sensors 18, no. 8: 2357. https://doi.org/10.3390/s18082357





