Reduced Graphene Oxide-Based Double Network Polymeric Hydrogels for Pressure and Temperature Sensing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of GO, CMC/rGO, and DN Hydrogels
2.3. Compressibility of Hydrogels
2.4. Pressure and Temperature Sensing
2.5. Characterization Techniques
3. Results and Discussion
3.1. Characterizations of GO and CMC/rGO
3.2. Morphological Characterizations of PNIPAm/CMC/rGO DN Hydrogels
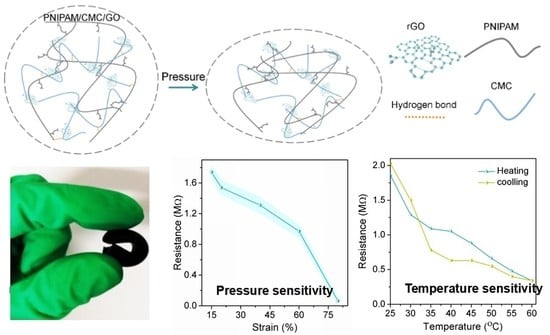
3.3. Compressibility and Pressure Sensing
3.4. Temperature Sensing
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Albertsson, A.; Voepel, J.; Edlund, U.; Dahlman, O.; Söderqvist-Lindblad, M. Design of renewable hydrogel release systems from fiberboard mill wastewater. Biomacromolecules 2010, 11, 1406–1411. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, S.; Endo, H.; Karino, T.; Haraguchi, K.; Shibayama, M. Gelation Mechanism of Poly(N-isopropylacrylamide)-Clay Nanocomposite Gels. Macromolecules 2007, 40, 4287–4295. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Liu, W. Dipole–Dipole and H-Bonding Interactions Significantly Enhance the Multifaceted Mechanical Properties of Thermoresponsive Shape Memory Hydrogels. Adv. Funct. Mater. 2015, 25, 471–480. [Google Scholar] [CrossRef]
- Lin, C.; Gitsov, I. Preparation and characterization of novel amphiphilic hydrogels with covalently attached drugs and fluorescent markers. Macromolecules 2010, 43, 10017–10030. [Google Scholar] [CrossRef]
- Söntjens, S.H.M.; Nettles, D.L.; Carnahan, M.A.; Setton, L.A.; Grinstaff, M.W. Biodendrimer-based hydrogel scaffolds for cartilage tissue repair. Biomacromolecules 2006, 7, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, B.; Banerjee, R. Biopolymer-based hydrogels for cartilage tissue engineering. Chem. Rev. 2011, 111, 4453–4474. [Google Scholar] [CrossRef] [PubMed]
- Lake, G.J.; Thomas, A.G. The strength of highly elastic materials. Proc. R. Soc. Lond. A 1967, 300, 108–119. [Google Scholar] [CrossRef]
- Naficy, S.; Brown, H.R.; Razal, J.M.; Spinks, G.M. Progress toward robust polymer hydrogels. Aust. J. Chem. 2011, 64, 1007–1025. [Google Scholar] [CrossRef]
- Fung, Y.C. Biomechanics: Mechanical Properties of Living Tissues, 2nd ed.; Springer: Berlin, Germany, 2013. [Google Scholar]
- Mente, P.L.; Lewis, J.L. Elastic modulus of calcified cartilage is an order of magnitude less than that of subchondral bone. J. Orthop. Res. 1994, 12, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-network hydrogels with extremely high mechanical strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Gong, J.P. Why are double network hydrogels so tough? Soft Matter 2010, 6, 2583–2590. [Google Scholar] [CrossRef]
- Haque, M.A.; Kurokawa, T.; Kamita, G.; Gong, J.P. Lamellar bilayers as reversible sacrificial bonds to toughen hydrogel: Hysteresis, self-recovery, fatigue resistance, and crack blunting. Macromolecules 2011, 44, 8916–8924. [Google Scholar] [CrossRef]
- Hu, J.; Hiwatashi, K.; Kurokawa, T.; Liang, S.M.; Wu, Z.L.; Gong, J.P. Microgel-reinforced hydrogel films with high mechanical strength and their visible mesoscale fracture structure. Macromolecules 2011, 44, 7775–7781. [Google Scholar] [CrossRef]
- Suekama, T.C.; Hu, J.; Kurokawa, T.; Gong, J.P.; Gehrke, S.H. Double-network strategy improves fracture properties of chondroitin sulfate networks. ACS Macro Lett. 2013, 2, 137–140. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, H.; Zhu, L.; Zheng, J. Fundamentals of double network hydrogels. J. Mater. Chem. B 2015, 3, 3654–3676. [Google Scholar] [CrossRef]
- Nakajima, T.; Furukawa, H.; Tanaka, Y.; Kurokawa, T.; Gong, J.P. Effect of void structure on the toughness of double network hydrogels. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 1246–1254. [Google Scholar] [CrossRef] [Green Version]
- Waters, D.J.; Engberg, K.; Parke-Houben, R.; Ta, C.N.; Jackson, A.J.; Toney, M.F.; Frank, C.W. Structure and mechanism of strength enhancement in interpenetrating polymer network hydrogels. Macromolecules 2011, 44, 5776–5787. [Google Scholar] [CrossRef]
- Huang, P.; Chen, W.; Yan, L. An inorganic–organic double network hydrogel of graphene and polymer. Nanoscale 2013, 5, 6034–6039. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, T.; Yu, X.; Wu, D.; Su, Z. Fabrication of Graphene–Biomacromolecule Hybrid Materials for Tissue Engineering Application. Polym. Chem. 2017, 8, 4309–4321. [Google Scholar] [CrossRef]
- Ding, J.; Sun, W.; Wei, G.; Su, Z. Cuprous Oxide Microspheres on Graphene Nanosheets: An Enhanced Material for Non-Enzymatic Electrochemical Detection of H2O2 and Glucose. RSC Adv. 2015, 5, 35338–35345. [Google Scholar] [CrossRef]
- Yu, X.; Liu, W.; Deng, X.; Yan, S.; Su, Z. Gold Nanocluster Embedded Bovine Serum Albumin Nanofibers-Graphene Hybrid Membranes for the Efficient Detection and Separation of Mercury Ion. Chem. Eng. J. 2018, 335, 176–184. [Google Scholar] [CrossRef]
- Emadi, F.; Amini, A.; Gholami, A.; Ghasemi, Y. Functionalized graphene oxide with chitosan for protein nanocarriers to protect against enzymatic cleavage and retain collagenase activity. Sci. Rep. 2017, 7, 42258–42270. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Lv, Y.; Williams, G.R.; Tao, L.; Yang, H.; Li, H.; Zhu, L. Lactobionic acid and Carboxymethyl Chitosan Functionalized Graphene Oxide Nanocomposites as Targeted Anticancer Drug Delivery Systems. Carbohydr. Polym. 2016, 151, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Chuang, W.Y.; Yang, S.Y.; Wu, W.J.; Lin, C.T. A Room-Temperature Operation Formaldehyde Sensing Material Printed Using Blends of Reduced Graphene Oxide and Poly(methyl methacrylate). Sensors 2015, 15, 28842–28853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Bremner, D.H.; Tao, L.; Li, H.; Hu, J.; Zhu, L. Carboxymethyl Chitosan-Mediated Synthesis of hyaluronic Acid-Targeted Graphene Oxide for Cancer Drug Delivery. Carbohydr. Polym. 2016, 135, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Yan, L. Supramolecular Hydrogel of Chitosan in the Presence of Graphene Oxide Nanosheets as 2D Cross-Linkers. ACS Sustain. Chem. Eng. 2013, 2, 296–300. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Y.; Wang, J.; Li, H.; Zhao, M.; Zhang, H.; Guan, Y.; Huang, H.; Mi, B.; Zhang, Y. Partially reduced graphene oxide and chitosan nanohybrid membranes for selective retention of divalent cations. RSC Adv. 2018, 8, 13656–13663. [Google Scholar] [CrossRef]
- Vusa, C.; Berchmans, S.; Alwarappan, S. Facile and green synthesis of graphene. RSC Adv. 2014, 4, 22470–22475. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, X.; Zhang, S.; Lu, X.; Li, Q.; Su, Z.; Wei, G. One-pot Green Synthesis, Characterizations, and Biosensor Application of Self-Assembled Reduced Graphene Oxide-Gold Nanoparticle Hybrid Membranes. J. Mater. Chem. B 2013, 1, 6525–6531. [Google Scholar] [CrossRef]
- Zhang, P.; Huang, Y.; Lu, X.; Zhang, S.; Li, J.; Wei, G.; Su, Z. One-step Synthesis of Large-Scale Graphene Film Doped with Gold Nanoparticles at Liquid-Air Interface for Electrochemistry and Raman Detection Applications. Langmuir 2014, 30, 8980–8989. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Ma, C.; Peng, L.; Yu, G. Conductive “smart” hybrid hydrogels with PNIPAM and nanostructured conductive polymers. Adv. Funct. Mater. 2015, 25, 1219–1225. [Google Scholar] [CrossRef]
- Haq, M.A.; Su, Y.; Wang, D. Mechanical properties of PNIPAM based hydrogels: A review. Mater. Sci. Eng. C 2017, 70, 842–855. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zeng, M.; Yu, Y.H.; Wang, H.; Mannan, M.S.; Cheng, Z. Thermosensitive ZrP-PNIPAM Pickering Emulsifier and the Controlled-Release Behavior. ACS Appl. Mater. Interfaces 2017, 9, 7852–7858. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Lewis, E.; Brambilla, G.; Wang, P. Temperature Sensing Performance of Microsphere Resonators. Sensors 2018, 18, 2515. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, P.; Yuan, Q.; Xu, X.; Lei, P.; Liu, X.; Su, Y.; Dong, L.; Feng, J.; Zhang, H. Nd3+-sensitized NaLuF4 luminescent nanoparticles for multimodal imaging and temperature sensing under 808 nm excitation. Nanoscale 2015, 7, 17861–17870. [Google Scholar] [CrossRef] [PubMed]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Zhang, X.; Wei, G.; Su, Z. Reduced Graphene Oxide-Based Double Network Polymeric Hydrogels for Pressure and Temperature Sensing. Sensors 2018, 18, 3162. https://doi.org/10.3390/s18093162
Liu W, Zhang X, Wei G, Su Z. Reduced Graphene Oxide-Based Double Network Polymeric Hydrogels for Pressure and Temperature Sensing. Sensors. 2018; 18(9):3162. https://doi.org/10.3390/s18093162
Chicago/Turabian StyleLiu, Wei, Xiaoyuan Zhang, Gang Wei, and Zhiqiang Su. 2018. "Reduced Graphene Oxide-Based Double Network Polymeric Hydrogels for Pressure and Temperature Sensing" Sensors 18, no. 9: 3162. https://doi.org/10.3390/s18093162