Preparation of Graphite Oxide Containing Different Oxygen-Containing Functional Groups and the Study of Ammonia Gas Sensitivity
Abstract
:1. Introduction
2. Experimental
2.1. Raw Materials and Reagents
2.2. Sample Preparation
2.3. Characterization
3. Results and Discussion
3.1. Color and Dispersion
3.2. Contact Angle
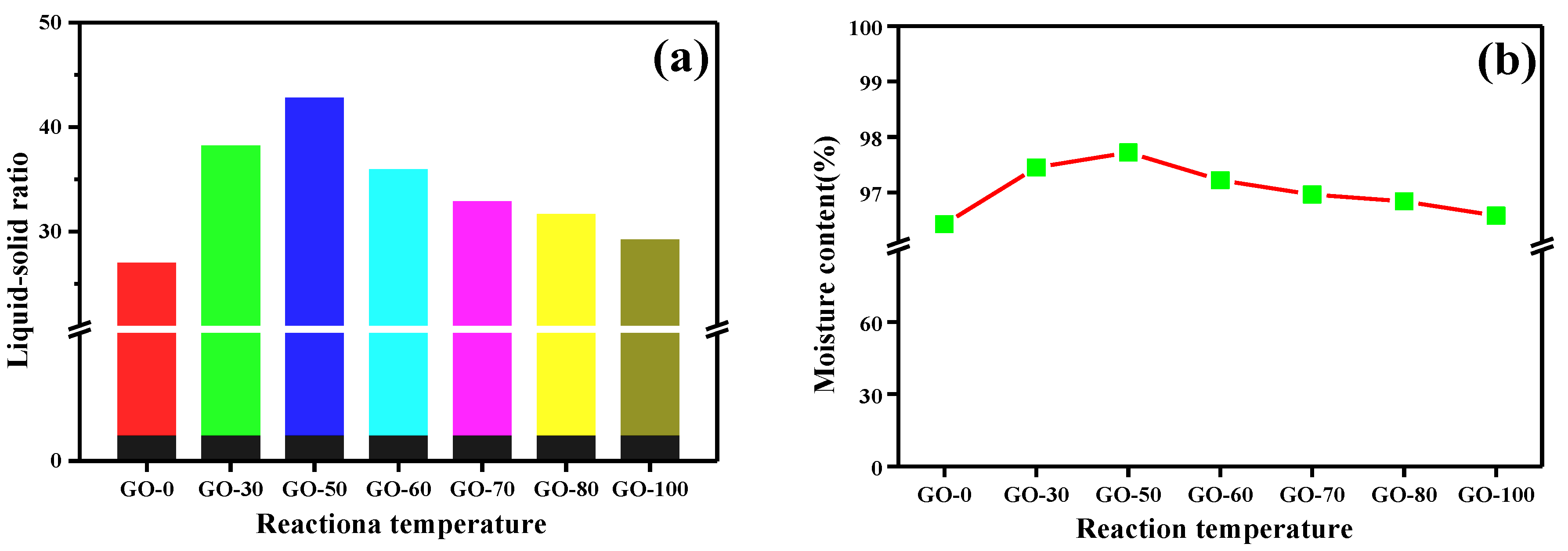
3.3. Moisture Content and Liquid-Solid Ratio
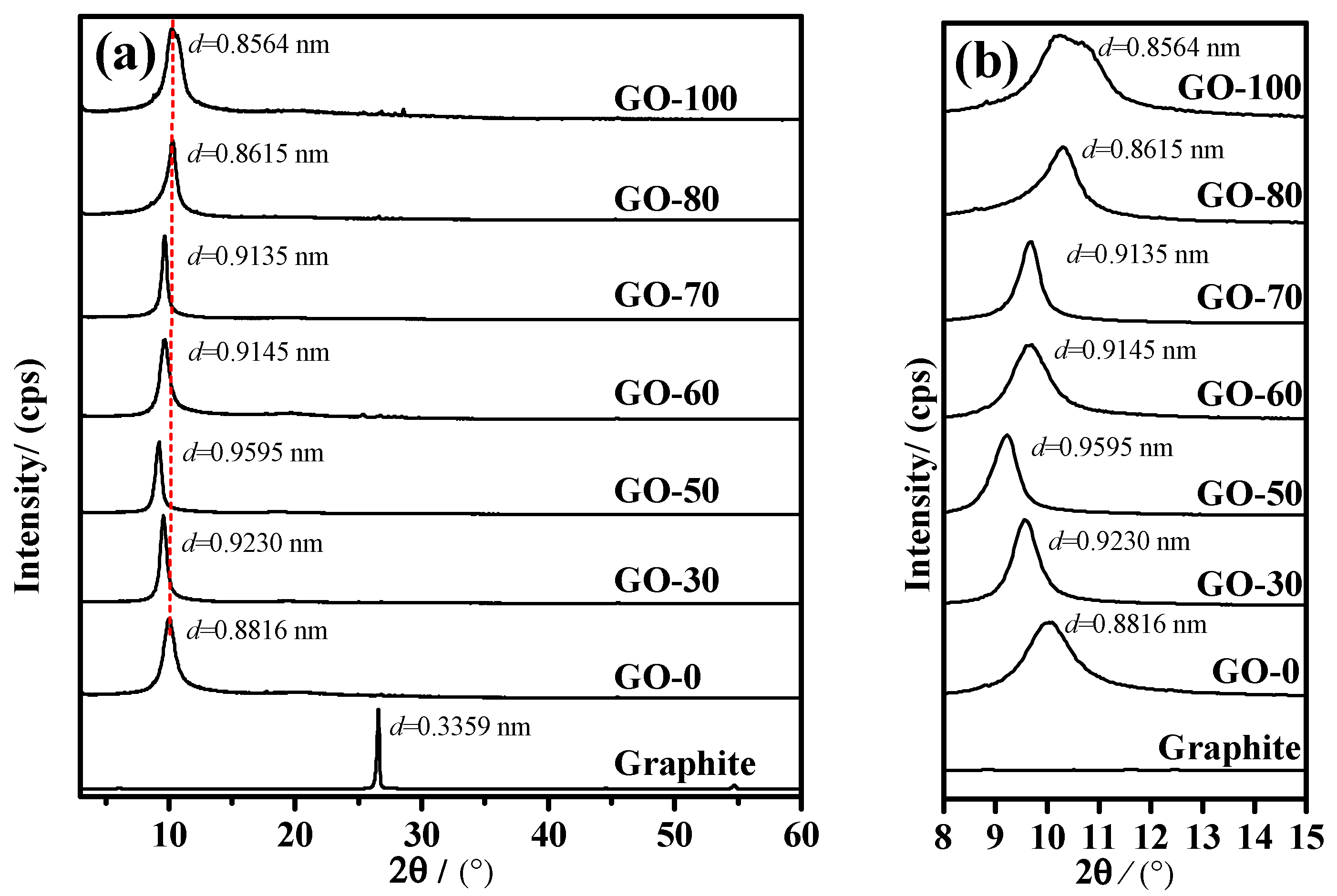
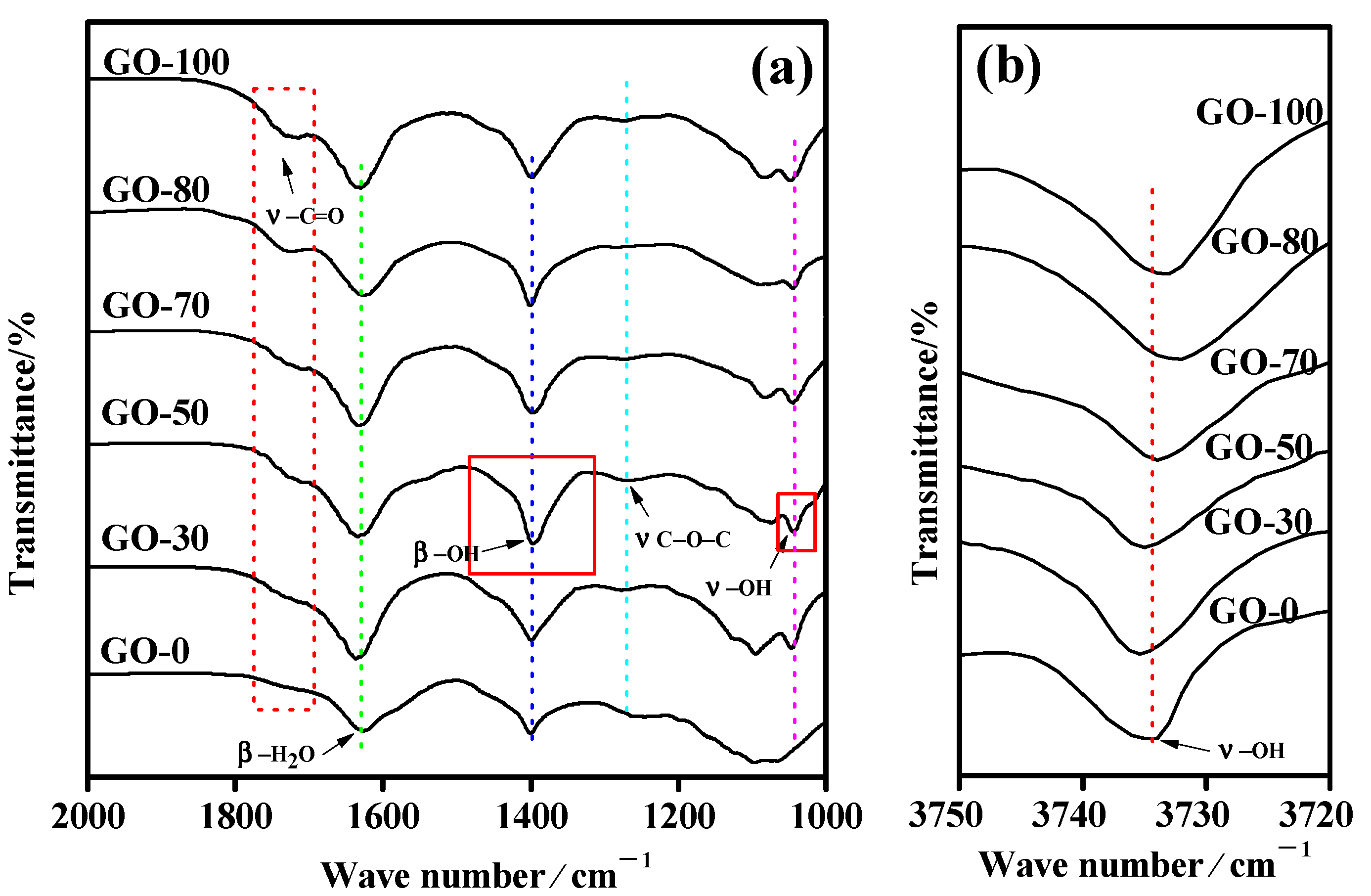
3.4. Structural Characterization
3.5. Gas Sensitivity Test
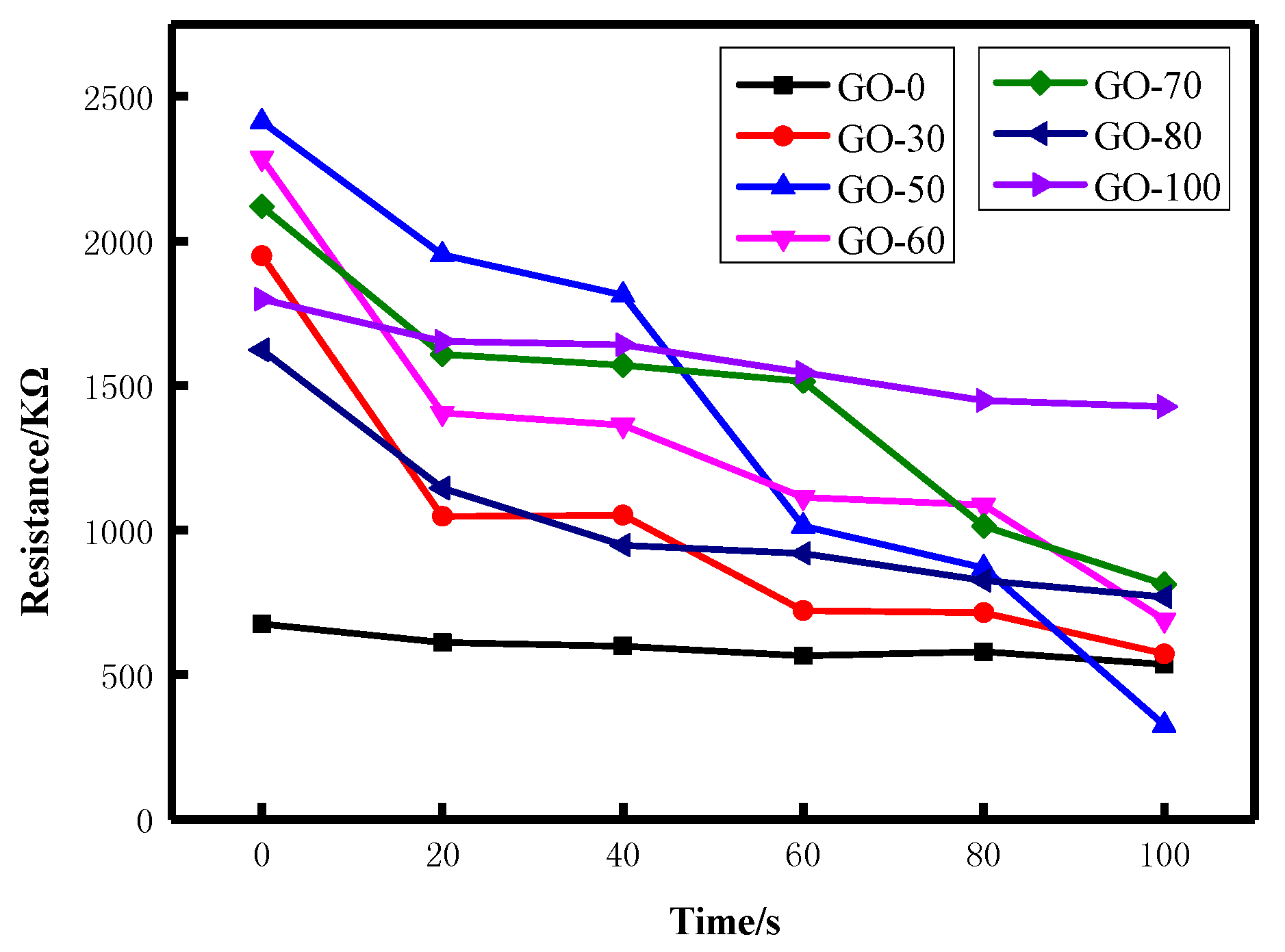
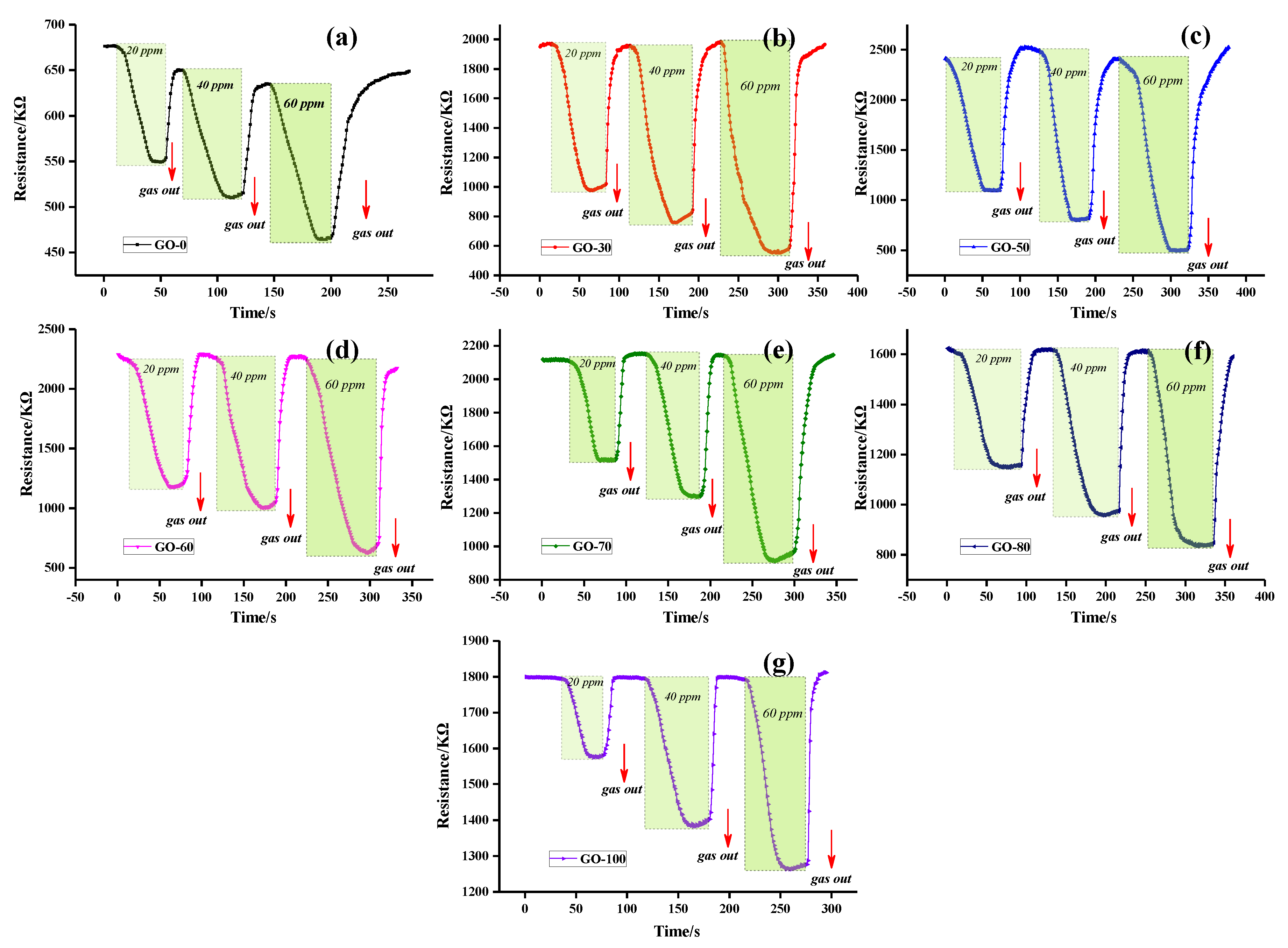
3.5.1. Static Resistance Analysis
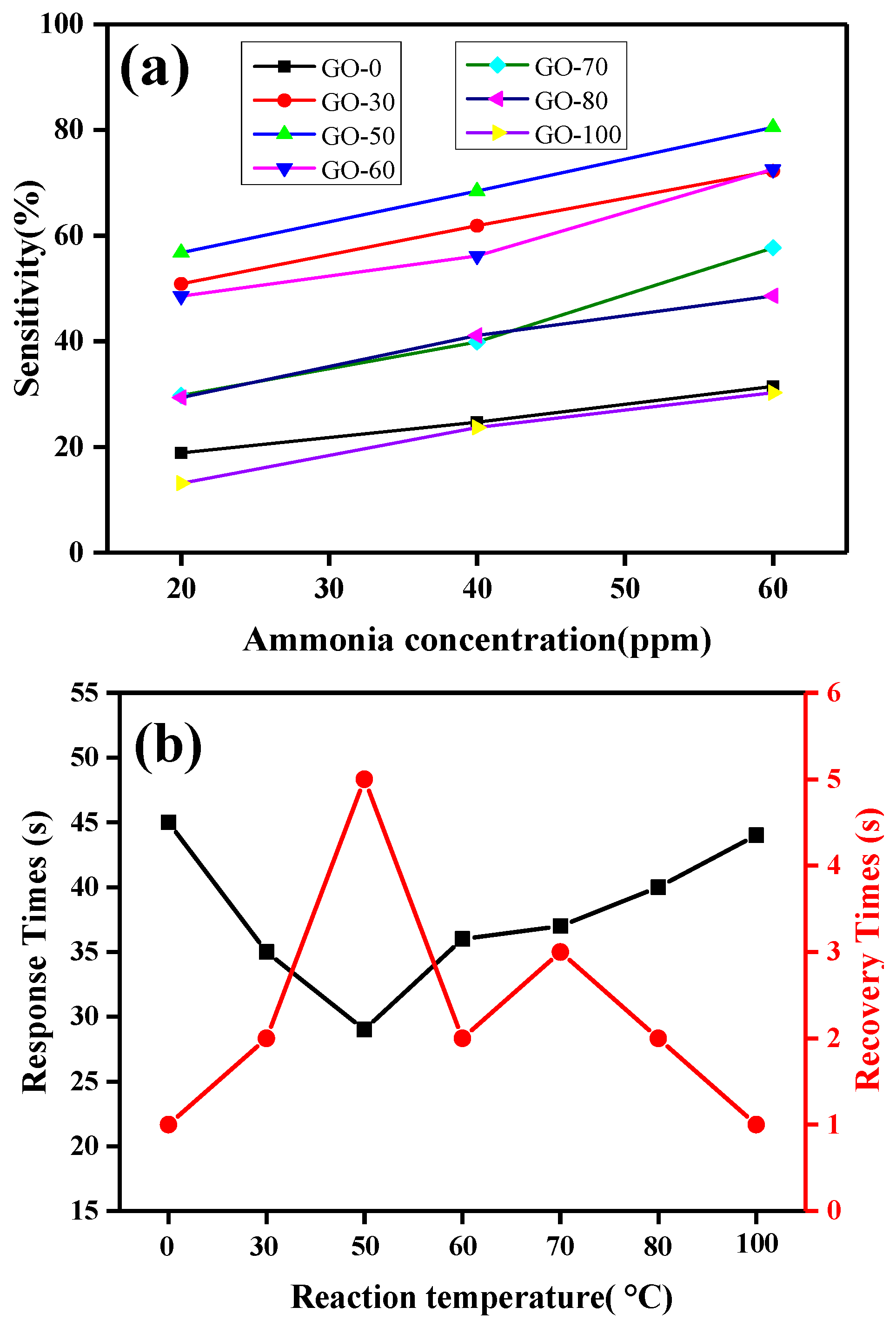
3.5.2. Dynamic Resistance and Sensitivity Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ohta, T.; Bostwick, A.; Mcchesney, J.; Seyller, T.; Horn, K.; Rotenberg, E. Controlling the Electronic Structure of Bilayer Graphene. Science 2006, 313, 951–954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, R.; Fujita, M.; Dresselhaus, G.; Dresselhaus, M.S. Electronic structure of chiral graphene tubules. Appl. Phys. Lett. 1992, 60, 2204–2206. [Google Scholar] [CrossRef]
- Mkhoyan, K.A.; Contryman, A.W.; Silcox, J.; Stewart, D.A.; Eda, G.; Mattevi, C.; Miller, S.; Chhowalla, M. Atomic and Electronic Structure of Graphene-Oxide. Microsc. Microanal. 2009, 16, 1058–1063. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tao, L.; Chen, Z.; Fang, H.; Li, X.; Wang, X.; Xu, J.B.; Zhu, H. Graphene and related two-dimensional materials: Structure-property relationships for electronics and optoelectronics. Appl. Phys. Rev. 2017, 4, 666. [Google Scholar] [CrossRef]
- Falkovsky, L.A. Optical properties of graphene. J. Exp. Theor. Phys. 2008, 115, 12004. [Google Scholar] [CrossRef]
- Pedersen, T.G.; Flindt, C.; Pedersen, J.; Jauho, A.P.; Mortensen, N.A.; Pedersen, K. Optical properties of graphene antidot lattices. Phys. Rev. B Condens. Matter 2009, 77, 245431. [Google Scholar] [CrossRef]
- Bonaccorso, F.; Sun, Z.; Hasan, T.; Ferrari, A.C. Graphene photonics and optoelectronics. Nat. Photonics 2010, 4, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Zu, Y.; Tang, J.; Zhu, W.; Zhang, M.; Liu, G.; Liu, Y.; Zhang, W.; Jia, M. Graphite oxide-supported CaO catalysts for transesterification of soybean oil with methanol. Bioresour. Technol. 2011, 102, 8939–8944. [Google Scholar] [CrossRef] [PubMed]
- Gao, W. The Chemistry of Graphene Oxide; Springer International Publishing: Berlin, Germany, 2015; pp. 61–95. [Google Scholar]
- Lobato, B.; Wendelbo, R.; Barranco, V.; Centeno, T.A. Graphite Oxide: An Interesting Candidate for Aqueous Supercapacitors. Electrochim. Acta 2014, 149, 245–251. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Zhai, S.; Zhai, Y.; Zhang, K.; Yue, Q.; Wang, L.; Zhao, J.; Wang, H.; Liu, J.; Jia, J. Preparation of sulfonic-functionalized graphene oxide as ion-exchange material and its application into electrochemiluminescence analysis. Biosens. Bioelectron. 2011, 26, 3136–3141. [Google Scholar] [CrossRef] [PubMed]
- Hontoria-Lucas, C.; López-Peinado, A.J.; López-González, J.D.D.; Rojas-Cervantes, M.L.; Martín-Aranda, R.M. Study of oxygen-containing groups in a series of graphite oxides: Physical and chemical characterization. Carbon 1995, 33, 1585–1592. [Google Scholar] [CrossRef]
- Huang, Q.; Sun, H.J.; Yang, Y.H. Spectroscopy Characterization and Analysis of Graphite Oxide. Chin. J. Inorg. Chem. 2011, 27, 1721–1726. [Google Scholar]
- Zhao, X.; Johnson, J.K. An Effective Potential for Adsorption of Polar Molecules on Graphite. Mol. Simul. 2005, 31, 1–10. [Google Scholar] [CrossRef]
- Zhang, B.; Li, F.; Wu, T.; Sun, D.; Li, Y. Adsorption of p-nitrophenol from aqueous solutions using nanographite oxide. Colloids Surf. A Physicochem. Eng. Asp. 2015, 464, 78–88. [Google Scholar] [CrossRef]
- Hsiao, M.C.; Liao, S.H.; Yen, M.Y.; Liu, P.I.; Pu, N.W.; Wang, C.A.; Ma, C.C. Preparation of covalently functionalized graphene using residual oxygen-containing functional groups. ACS Appl. Mater. Interfaces 2010, 2, 3092. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, Z.; Huang, Z.; Zhang, H. Effects of oxygen-containing functional groups on the supercapacitor performance of incompletely reduced graphene oxides. Int. J. Hydrogen Energy 2016, 42, 7186–7194. [Google Scholar] [CrossRef]
- Wang, S.; Cole, I.S.; Zhao, D.; Li, Q. The dual roles of functional groups in the photoluminescence of graphene quantum dots. Nanoscale 2016, 8, 7449–7458. [Google Scholar] [CrossRef] [PubMed]
- Staudenmaier, L.; Dtsch, B. The structure of graphite oxide: Investigation of its surface chemical groups. Chem. Ges 1898, 31, 1481–1487. [Google Scholar] [CrossRef]
- Nakajima, T.; Matsuo, Y. Formation process and structure of graphite oxide. Carbon 1994, 32, 469–475. [Google Scholar] [CrossRef]
- Nakajima, T.; Mabuchi, A.; Hagiwara, R. A new structure model of graphite oxide. Carbon 1988, 26, 357–361. [Google Scholar] [CrossRef]
- Beckett, R.J.; Croft, R.C. The Structure of Graphite Oxide. J. Phys. Chem. 1952, 56, 929–941. [Google Scholar] [CrossRef]
- Mermoux, M.; Chabre, Y.; Rousseau, A. FTIR and 13 C NMR study of graphite oxide. Carbon 1991, 29, 469–474. [Google Scholar] [CrossRef]
- Avdeev, V.V.; Sorokina, N.E.; Nikol’ Skaya, I.V.; Monyakina, L.A.; Voronkina, A.V. Synthesis of Intercalation Compounds in the System Graphite-HNO3-H2SO4. Inorg. Mater. 1997, 33, 584–587. [Google Scholar]
- Vieira, M.A.; Gonçalves, G.R.; Cipriano, D.F.; Schettino, M.A., Jr.; Filho, E.A.S.; Cunha, A.G.; Emmerich, F.G.; Freitas, J.C.C. Synthesis of graphite oxide from milled graphite studied by solid-state 13 C nuclear magnetic resonance. Carbon 2016, 98, 496–503. [Google Scholar] [CrossRef]
- Hamwi, A.; Marchand, V. Some chemical and electrochemical properties of graphite oxide. J. Phys. Chem. Solids 1996, 57, 867–872. [Google Scholar] [CrossRef]
- Jeong, H.K.; Lee, Y.P.; Lahaye, R.J.; Park, M.H.; An, K.H.; Kim, I.J.; Yang, C.W.; Park, C.Y.; Ruoff, R.S.; Lee, Y.H. Evidence of Graphitic AB Stacking Order of Graphite Oxides. J. Am. Chem. Soc. 2008, 130, 1362–1366. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Peng, W.; Li, Y.; Li, X.; Wang, S.; Zhang, G.; Zhang, F. Deoxygenation of Exfoliated Graphite Oxide under Alkaline Conditions: A Green Route to Graphene Preparation. Adv. Mater. 2008, 20, 4490–4493. [Google Scholar] [CrossRef]
- Boehm, H.P.; Clauss, A.; Hofmann, U. Graphite oxide and its membrane properties. J. Chim. Phys. 1961, 58, 141–147. [Google Scholar] [CrossRef]
- He, H.; Riedl, T.; Lerf, A.; Klinowski, J. Solid-State NMR Studies of the Structure of Graphite Oxide. J. Phys. Chem. 1996, 100, 19954–19958. [Google Scholar] [CrossRef]
- He, H.; Klinowski, J.; Forster, M.; Lerf, A. A new structural model for graphite oxide. Chem. Phys. Lett. 1998, 287, 53–56. [Google Scholar] [CrossRef]
- Lerf, A.; He, H.; Forster, M.; Klinowski, J. Structure of Graphite Oxide Revisited. J. Phys. Chem. B 1998, 102, 4477–4482. [Google Scholar] [CrossRef]
- Lerf, A.; Buchsteiner, A.; Pieper, J.; Schöttl, S.; Dekany, I.; Szabo, T.; Boehm, H.P. Hydration behavior and dynamics of water molecules in graphite oxide. J. Phys. Chem. Solids 2006, 67, 1106–1110. [Google Scholar] [CrossRef]
- Dékány, I.; Krüger-Grasser, R.; Weiss, A. Selective liquid sorption properties of hydrophobized graphite oxide nanostructures. Colloid Polym. Sci. 1998, 276, 570–576. [Google Scholar]
- Ramesh, P.; Bhagyalakshmi, S.; Sampath, S. Preparation and physicochemical and electrochemical characterization of exfoliated graphite oxide. J. Colloid Interface Sci. 2004, 274, 95. [Google Scholar] [CrossRef] [PubMed]
- Peckett, J.W.; Trens, P.; Gougeon, R.D.; Pöppl, A.; Harris, R.K.; Hudson, M.J. Electrochemically oxidised graphite: Characterisation and some ion exchange properties. Carbon 2000, 38, 345–353. [Google Scholar] [CrossRef]
- De Wit, M.; Vanneste, E.; Blockhuys, F.; Verreyt, G.; Tachelet, W.; Nagels, L.J.; Geise, H.J. Chemically Sensitive Sensor Comprising Arylene Alkenylene Oligomers. U.S. Patent 6,042,788, 28 March 2000. [Google Scholar]
- Drewniak, S.; Muzyka, R.; Stolarczyk, A.; Pustelny, T.; Kotyczka-Moraå Ska, M.; Setkiewicz, M. Studies of Reduced Graphene Oxide and Graphite Oxide in the Aspect of Their Possible Application in Gas Sensors. Sensors 2016, 16, 103. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kwak, Y.; Lee, I.Y.; Maeng, S.; Kim, G.H. Highly responsive hydrogen gas sensing by partially reduced graphite oxide thin films at room temperature. Carbon 2012, 50, 4061–4067. [Google Scholar] [CrossRef]
- Yeh, T.F.; Syu, J.M.; Cheng, C.; Chang, T.H.; Teng, H. Graphite Oxide as a Photocatalyst for Hydrogen Production from Water. Adv. Funct. Mater. 2010, 20, 2255–2262. [Google Scholar] [CrossRef]
- Xu, C.; Cao, Y.; Kumar, R.; Wu, X.; Wang, X.; Scott, K. A polybenzimidazole/sulfonated graphite oxide composite membrane for high temperature polymer electrolyte membrane fuel cells. J. Mater. Chem. 2011, 21, 11359–11364. [Google Scholar] [CrossRef]
- Travlou, N.A.; Kyzas, G.Z.; Lazaridis, N.K.; Deliyanni, E.A. Graphite oxide/chitosan composite for reactive dye removal. Chem. Eng. J. 2013, 217, 256–265. [Google Scholar] [CrossRef]
- Zhao, Y.; Tang, G.S.; Yu, Z.Z.; Qi, J.S. The effect of graphite oxide on the thermoelectric properties of polyaniline. Carbon 2012, 50, 3064–3073. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, H.; Peng, T. Structure Development during the Cation Exchange Processes of Graphite Oxide. Acta Phys. -Chim. 2016, 33, 413–418. [Google Scholar]
- Wang, P.; Sun, H.; Peng, T. The Evolution Rule of Three-Dimensional Structures of Graphite During Oxidation. Nano Brief Rep. Rev. 2015, 10, 1550014. [Google Scholar] [CrossRef]
- Sun, H.; Peng, T. The Preparation of Graphene Materials via Oxidation-Reduction; Science Press: Henderson, NV, USA, 2015. [Google Scholar]
- Cotton, F.A.; Wilkinson, G. Advance Inorganic Chemistry. J. Chem. Educ. 1988, 9, 417–420. [Google Scholar]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Ding, J.N.; Liu, Y.B.; Yuan, N.Y.; Ding, G.Q.; Fan, Y.; Yu, C.T. The influence of temperature, time and concentration on the dispersion of reduced graphene oxide prepared by hydrothermal reduction. Diam. Relat. Mater. 2012, 21, 11–15. [Google Scholar] [CrossRef]
- Lu, G.; Ocola, L.E.; Chen, J. Reduced graphene oxide for room-temperature gas sensors. Nanotechnology 2009, 20, 445–502. [Google Scholar] [CrossRef] [PubMed]
- Yue, P.; Li, J. Ammonia adsorption on graphene and graphene oxide: A first-principles study. Front. Environ. Sci. Eng. 2013, 7, 403–411. [Google Scholar]




| Sample | Relative Percentage Content /% | C/O | |||
|---|---|---|---|---|---|
| C=C | C−OH | Epoxy Groups | O−C=O | ||
| GO-0 | 53.75 | 23.08 | 18.03 | 5.14 | 2.09 |
| GO-30 | 43.80 | 28.77 | 19.95 | 7.48 | 1.90 |
| GO-50 | 42.58 | 30.01 | 19.72 | 7.69 | 1.85 |
| GO-60 | 42.23 | 27.92 | 21.33 | 8.52 | 1.86 |
| GO-70 | 44.78 | 25.27 | 21.40 | 8.54 | 1.89 |
| GO-80 | 45.42 | 21.33 | 22.93 | 10.31 | 2.06 |
| GO-100 | 45.83 | 20.18 | 22.96 | 11.03 | 2.23 |
| GO-0 | GO-30 | GO-50 | GO-60 | GO-70 | GO-80 | GO-100 | |
|---|---|---|---|---|---|---|---|
| Response time (s) | 45 | 35 | 29 | 36 | 37 | 40 | 44 |
| Recovery time (s) | 1 | 2 | 5 | 2 | 3 | 2 | 1 |
| Sensitivity value (%) | 18.895 | 50.867 | 56.795 | 48.539 | 29.742 | 29.346 | 13.123 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, L.; Peng, T.; Yuan, M.; Sun, H.; Dai, S.; Wang, L. Preparation of Graphite Oxide Containing Different Oxygen-Containing Functional Groups and the Study of Ammonia Gas Sensitivity. Sensors 2018, 18, 3745. https://doi.org/10.3390/s18113745
Luo L, Peng T, Yuan M, Sun H, Dai S, Wang L. Preparation of Graphite Oxide Containing Different Oxygen-Containing Functional Groups and the Study of Ammonia Gas Sensitivity. Sensors. 2018; 18(11):3745. https://doi.org/10.3390/s18113745
Chicago/Turabian StyleLuo, Liming, Tongjiang Peng, Mingliang Yuan, Hongjuan Sun, Shichan Dai, and Long Wang. 2018. "Preparation of Graphite Oxide Containing Different Oxygen-Containing Functional Groups and the Study of Ammonia Gas Sensitivity" Sensors 18, no. 11: 3745. https://doi.org/10.3390/s18113745




