Ammonium Fluoride Passivation of CdZnTeSe Sensors for Applications in Nuclear Detection and Medical Imaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Detector Material and Composition
2.2. Planar Detector Fabrication and Passivation
2.3. Current-Voltage, Charge-Transport, and Detector Energy Resolution Experiments
3. Results and Discussion
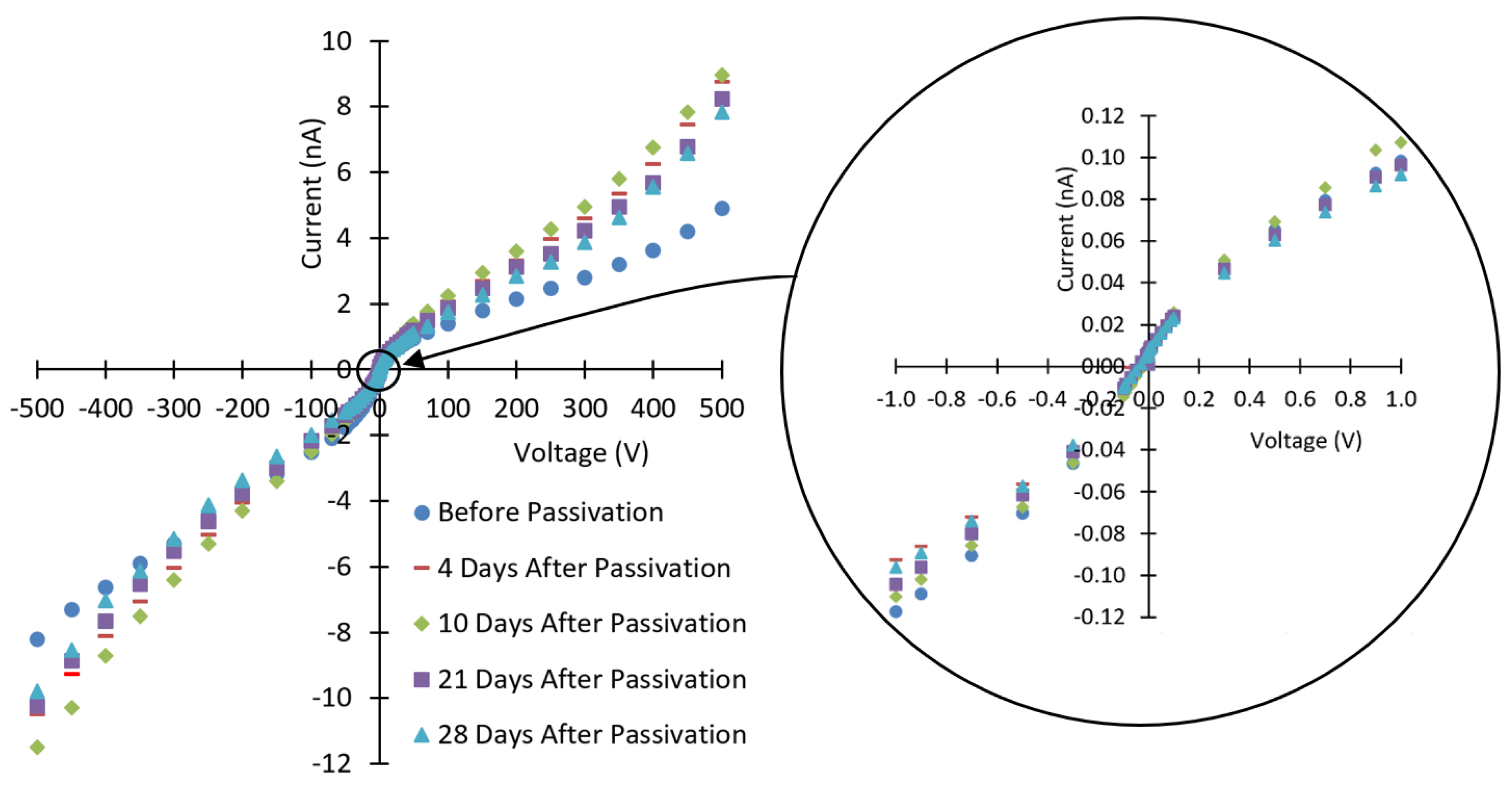
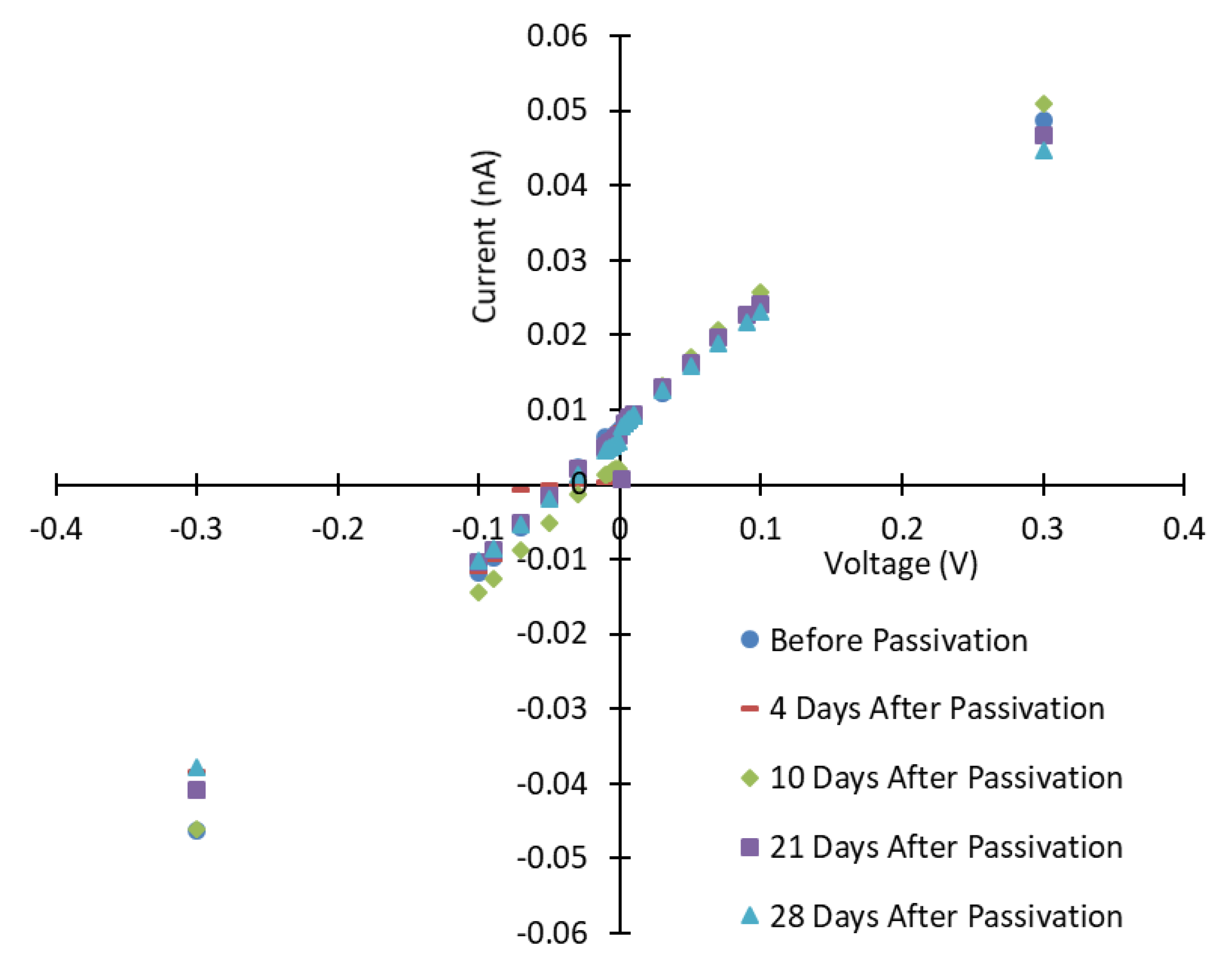
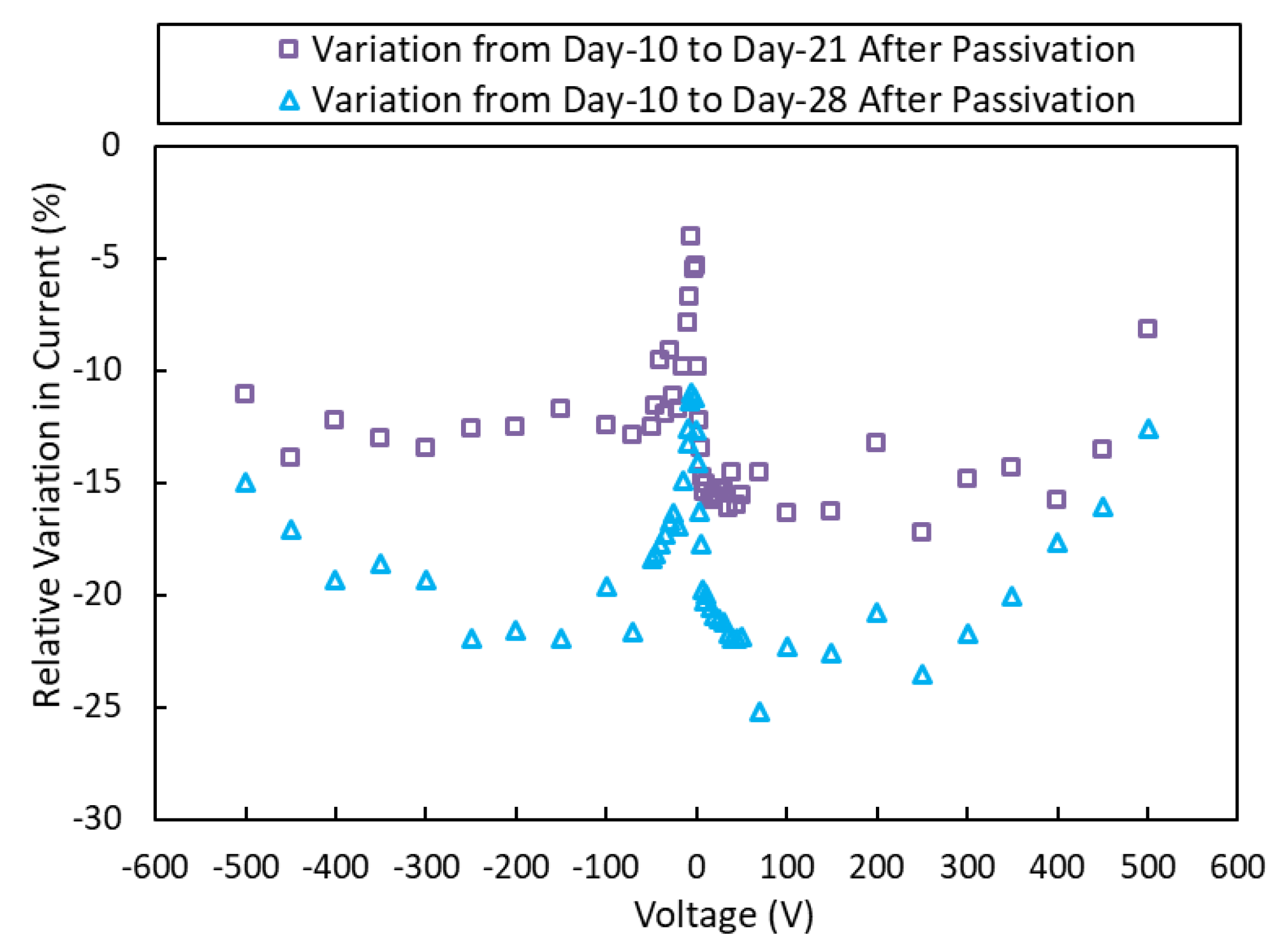
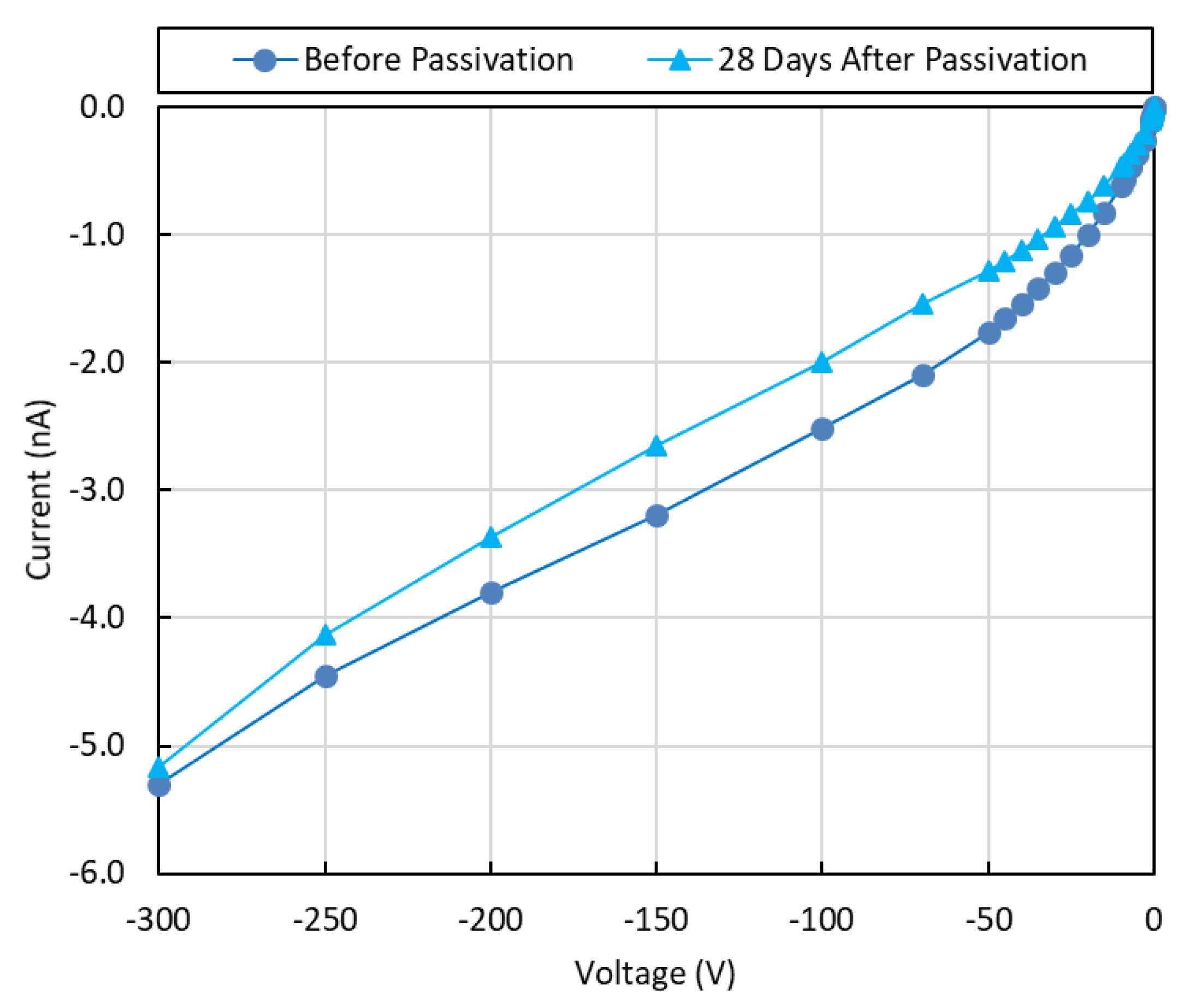
3.1. Current-Voltage Characteristics
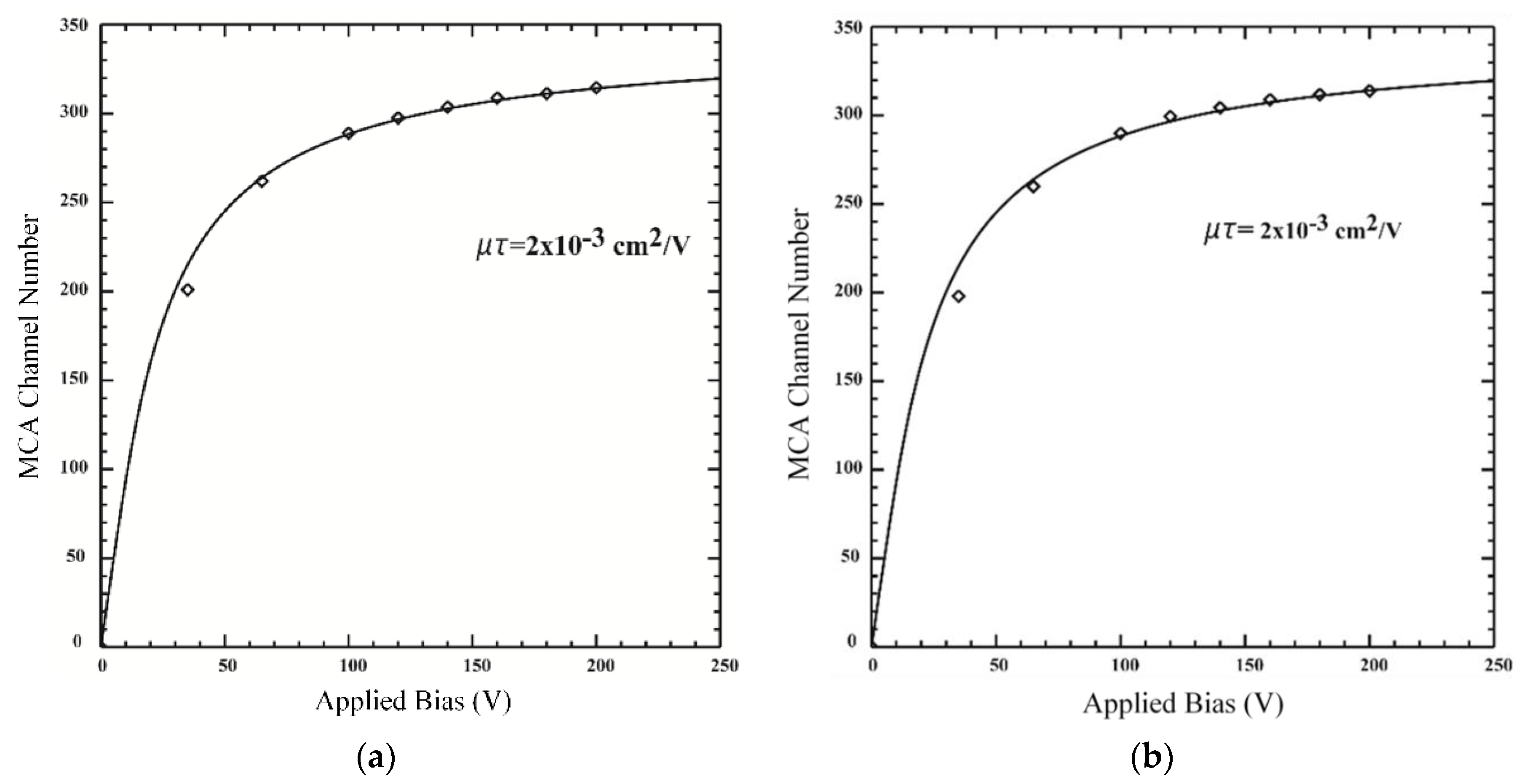
3.2. Charge Transport
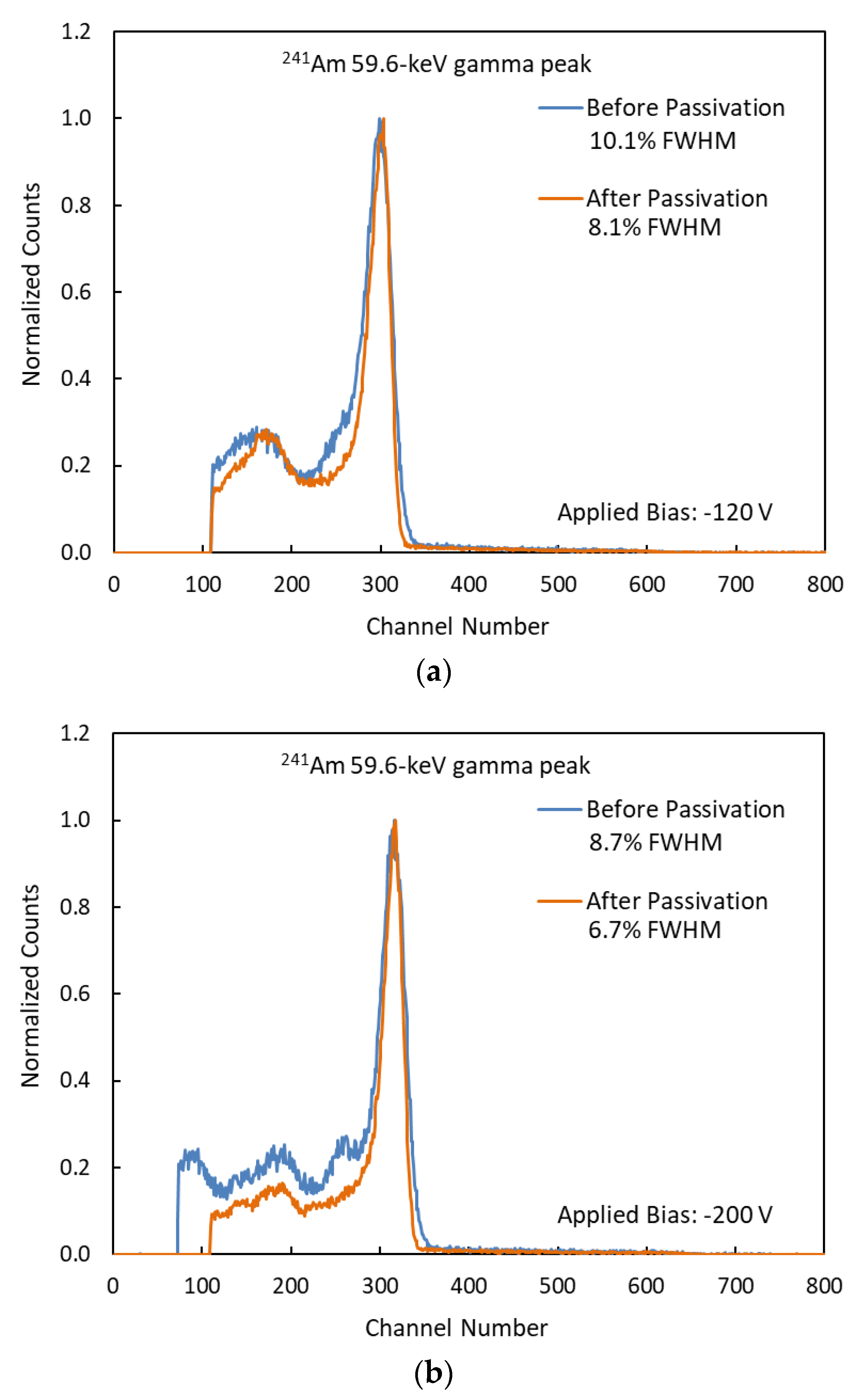
3.3. Energy Resolution
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roy, U.N.; Camarda, G.S.; Cui, Y.; Gul, R.; Hossain, A.; Yang, G.; Zazvorka, J.; Dedic, V.; Franc, J.; James, R.B. Role of selenium addition to CdZnTe matrix for room-temperature radiation detector applications. Sci. Rep. 2019, 9, 1620. [Google Scholar] [CrossRef] [PubMed]
- Rejhon, M.; Franc, J.; Dědič, V.; Pekárek, J.; Roy, U.N.; Grill, R.; James, R.B. Influence of deep levels on the electrical transport properties of CdZnTeSe detectors. J. Appl. Phys. 2018, 124, 235702. [Google Scholar] [CrossRef] [Green Version]
- Roy, U.N.; Camarda, G.S.; Cui, Y.; Gul, R.; Yang, G.; Hossain, A.; James, R.B.; Zazvorka, J.; Dedic, V.; Franc, J.; et al. Crystal growth of CdZnTeSe (CZTS) gamma detectors: a promising alternative to CdZnTe. In Hard X-Ray, Gamma-Ray, and Neutron Detector Physics XIX; International Society for Optics and Photonics: Bellingham, WA, USA, 2017; Volume 10392, p. 103920Y. [Google Scholar]
- Barber, H.B. Applications of semiconductor detectors to nuclear medicine. Nucl. Instrum. Methods Phys. Res. Sect. A 1999, 436, 102–110. [Google Scholar] [CrossRef]
- Wahl, C.G.; Kaye, W.R.; Wang, W.; Zhang, F.; Jaworski, J.M.; King, A.; Boucher, Y.A.; He, Z. The Polaris-H imaging spectrometer. Nucl. Instrum. Methods Phys. Res. Sect. A 2015, 784, 377–381. [Google Scholar] [CrossRef]
- Krawczynski, H.S.; Stern, D.; Harrison, F.A.; Kislat, F.F.; Zajczyk, A.; Beilicke, M.; Miyasaka, H. X-ray polarimetry with the polarization spectroscopic telescope array (PolSTAR). Astropart. Phys. 2016, 75, 8–28. [Google Scholar] [CrossRef]
- Iniewski, K. CZT sensors for Computed Tomography: from crystal growth to image quality. J. Instrum. 2016, 11, C12034. [Google Scholar] [CrossRef]
- Egarievwe, S.U.; Chan, W.; Kim, K.H.; Roy, U.N.; Sams, V.; Hossain, A.; Kassu, A.; James, R.B. Carbon Coating and defects in CdZnTe and CdMnTe nuclear detectors. IEEE Trans. Nucl. Sci. 2016, 63, 236–245. [Google Scholar] [CrossRef]
- Bolotnikov, A.E.; Babalola, S.; Camarda, G.S.; Cui, Y.; Egarievwe, S.U.; Hawrami, R.; Hossain, A.; Yang, G.; James, R.B. Te inclusions in CZT detectors: New method for correcting their adverse effects. IEEE Trans. Nucl. Sci. 2010, 57, 910–919. [Google Scholar] [CrossRef]
- Zhang, N.; Yeckel, A.; Burger, A.; Cui, Y.; Lynn, K.G.; Derby, J.J. Anomalous segregation during electrodynamic gradient freeze growth of cadmium zinc telluride. J. Cryst. Growth 2011, 325, 10–19. [Google Scholar] [CrossRef]
- Bolotnikov, A.E.; Camarda, G.S.; Cui, Y.; Yang, G.; Hossain, A.; Kim, K.; James, R.B. Characterization and evaluation of extended defects in CZT crystals for gamma-ray detectors. J. Cryst. Growth 2013, 379, 46–56. [Google Scholar] [CrossRef]
- Hossain, A.; Bolotnikov, A.E.; Camarda, G.S.; Cui, Y.; Yang, G.; Kim, K.H.; Gul, R.; Xu, L.; James, R.B. Extended defects in CdZnTe crystals: Effects on device performance. J. Cryst. Growth 2010, 312, 1795–1799. [Google Scholar] [CrossRef]
- Amman, M.; Lee, J.S.; Luke, P.N. Electron trapping nonuniformity in high-pressure-Bridgman-grown CdZnTe. J. Appl. Phys. 2002, 92, 3198–3206. [Google Scholar] [CrossRef] [Green Version]
- Roy, U.N.; Camarda, G.S.; Cui, Y.; James, R.B. High-resolution virtual Frisch grid gamma-ray detectors based on as-grown CdZnTeSe with reduced defects. Appl. Phys. Lett. 2019, 114, 232107. [Google Scholar] [CrossRef]
- Roy, U.N.; Camarda, G.S.; Cui, Y.; Gul, R.; Yang, G.; Zazvorka, J.; Dedic, V.; Franc, J.; James, R.B. Evaluation of CdZnTeSe as a high-quality gamma-ray spectroscopic material with better compositional homogeneity and reduced defects. Sci. Rep. 2019, 9, 7303. [Google Scholar] [CrossRef] [PubMed]
- Egarievwe, S.U.; Hossain, A.; Okwechime, I.O.; Egarievwe, A.A.; Jones, D.E.; Roy, U.N.; James, R.B. Effects of Chemical Treatments on CdZnTe X-Ray and Gamma-Ray Detectors. IEEE Trans. Nucl. Sci. 2016, 63, 1091–1098. [Google Scholar] [CrossRef]
- Egarievwe, S.U.; Hossain, A.; Okwechime, I.O.; Gul, R.; James, R.B. Effects of Chemomechanical Polishing on CdZnTe X-ray and Gamma-Ray Detectors. J. Electron. Mater. 2015, 44, 3194–3201. [Google Scholar] [CrossRef]
- Okwechime, I.O.; Egarievwe, S.U.; Hossain, A.; Hales, Z.M.; Egarievwe, A.A.; James, R.B. Chemical treatment of CdZnTe radiation detectors using hydrogen bromide and ammonium-based solutions. In Hard X-Ray, Gamma-Ray, and Neutron Detector Physics XVI; International Society for Optics and Photonics: Bellingham, WA, USA, 2014; Volume 9213, p. 92130Y. [Google Scholar]
- Egarievwe, S.U.; Jow, J.O.; Egarievwe, A.A.; Gul, R.; Martin, R.D.; Hales, Z.M.; Hossain, A.; Roy, U.N.; James, R.B. Effects of Etching and Chemo-Mechanical Polishing on the Electrical Properties of CdZnTe Nuclear Detectors. Am J. Mater. Sci. 2015, 5, 16–20. [Google Scholar]
- Chattopadhyay, K.; Hayes, M.; Ndap, J.O.; Burger, A.; Lu, W.J.; McWhinney, H.G.; Grady, T.; James, R.B. Surface passivation of cadmium zinc telluride radiation detectors by potassium hydroxide solution. J. Electron. Mater. 2000, 29, 708–712. [Google Scholar] [CrossRef]
- Babar, S.; Sellin, P.J.; Watts, J.F.; Baker, M.A. An XPS study of bromine in methanol etching and hydrogen peroxide passivation treatments for cadmium zinc telluride radiation detectors. Appl. Surf. Sci. 2013, 264, 681–686. [Google Scholar] [CrossRef] [Green Version]
- Hossain, A.; Cui, Y.G.; Bolotnikov, A.E.; Camarda, G.S.; Yang, G.; Kochanowska, D.; Witkowska-Baran, M.; Mycielski, A.; James, R.B. Vanadium-doped cadmium manganese telluride (Cd1−xMnxTe) crystals as x-and gamma-ray detectors. J. Electron. Mater. 2009, 38, 1593–1599. [Google Scholar] [CrossRef]
- Zambelli, N.; Marchini, L.; Benassi, G.; Calestani, D.; Caroli, E.; Zappettini, A. Electroless gold contact deposition on CdZnTe detectors by scanning pipette technique. J. Instrum. 2012, 7, P08022. [Google Scholar] [CrossRef]
- Zheng, Q.; Dierre, F.; Ayoub, M.; Crocco, J.; Bensalah, H.; Corregidor, V.; Alves, E.; Fernandez-Ruiz, R.; Perez, J.M.; Dieguez, E. Comparison of radiation detector performance for different metal contacts on CdZnTe deposited by electroless deposition method. Cryst. Res. Technol. 2011, 46, 1131–1136. [Google Scholar] [CrossRef]
- Nemirovsky, Y.; Ruzin, A.; Asa, G.; Gorelik, Y.; Li, L. Study of contacts to CdZnTe radiation detectors. J. Electron. Mater. 1997, 26, 756–764. [Google Scholar] [CrossRef]
- Bolotnikov, A.E.; Camarda, G.C.; Wright, G.W.; James, R.B. Factors limiting the performance of CdZnTe detectors. IEEE Trans. Nucl. Sci. 2005, 52, 589–598. [Google Scholar] [CrossRef]
- Wright, G.; Cui, Y.; Roy, U.N.; Barnett, C.; Reed, K.; Burger, A.; Lu, F.; Li, L.; James, R.B. The effects of chemical etching on the charge collection efficiency of {111} oriented Cd/sub 0.9/Zn/sub 0.1/Te nuclear radiation detectors. IEEE Trans. Nucl. Sci. 2002, 49, 2521–2525. [Google Scholar] [CrossRef]
- Pekarek, J.; Belas, E.; Zazvorka, J. Long-term stable surface treatments on CdTe and CdZnTe radiation detectors. J. Electron. Mater. 2017, 46, 1996–2002. [Google Scholar] [CrossRef]
| Applied Voltage (V) | FWHM before Passivation (%) | FWHM after Passivation (%) | Improvement in Energy Resolution |
|---|---|---|---|
| −35 | 17.9 | 12.0 | 33% |
| −65 | 12.9 | 10.0 | 22% |
| −100 | 9.9 | 8.0 | 19% |
| −120 | 10.1 | 8.1 | 20% |
| −140 | 10.0 | 7.2 | 28% |
| −160 | 9.3 | 6.9 | 26% |
| −180 | 8.9 | 6.4 | 28% |
| −200 | 8.7 | 6.7 | 23% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egarievwe, S.U.; Roy, U.N.; Goree, C.A.; Harrison, B.A.; Jones, J.; James, R.B. Ammonium Fluoride Passivation of CdZnTeSe Sensors for Applications in Nuclear Detection and Medical Imaging. Sensors 2019, 19, 3271. https://doi.org/10.3390/s19153271
Egarievwe SU, Roy UN, Goree CA, Harrison BA, Jones J, James RB. Ammonium Fluoride Passivation of CdZnTeSe Sensors for Applications in Nuclear Detection and Medical Imaging. Sensors. 2019; 19(15):3271. https://doi.org/10.3390/s19153271
Chicago/Turabian StyleEgarievwe, Stephen U., Utpal N. Roy, Carmella A. Goree, Benicia A. Harrison, Jeanette Jones, and Ralph B. James. 2019. "Ammonium Fluoride Passivation of CdZnTeSe Sensors for Applications in Nuclear Detection and Medical Imaging" Sensors 19, no. 15: 3271. https://doi.org/10.3390/s19153271




