Spectroscopic Understanding of SnO2 and WO3 Metal Oxide Surfaces with Advanced Synchrotron Based; XPS-UPS and Near Ambient Pressure (NAP) XPS Surface Sensitive Techniques for Gas Sensor Applications under Operational Conditions
Abstract
:1. Introduction
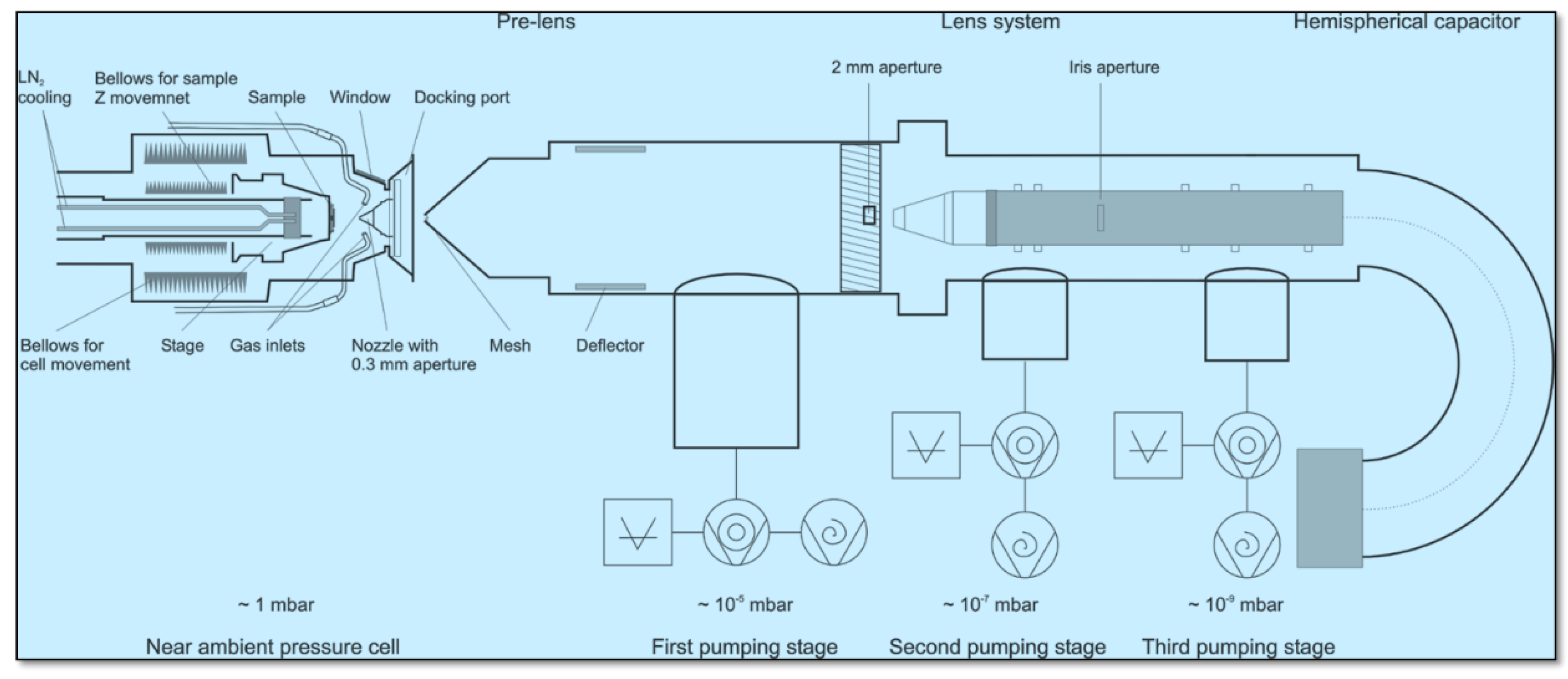
2. Materials and Methods
3. Results
3.1. Syncrothon Based XPS Analysis of WO3 for Precise Positioning of Chemisorbed Oxygen Species and Some Remarks on Oxidation State Analysis of WO3
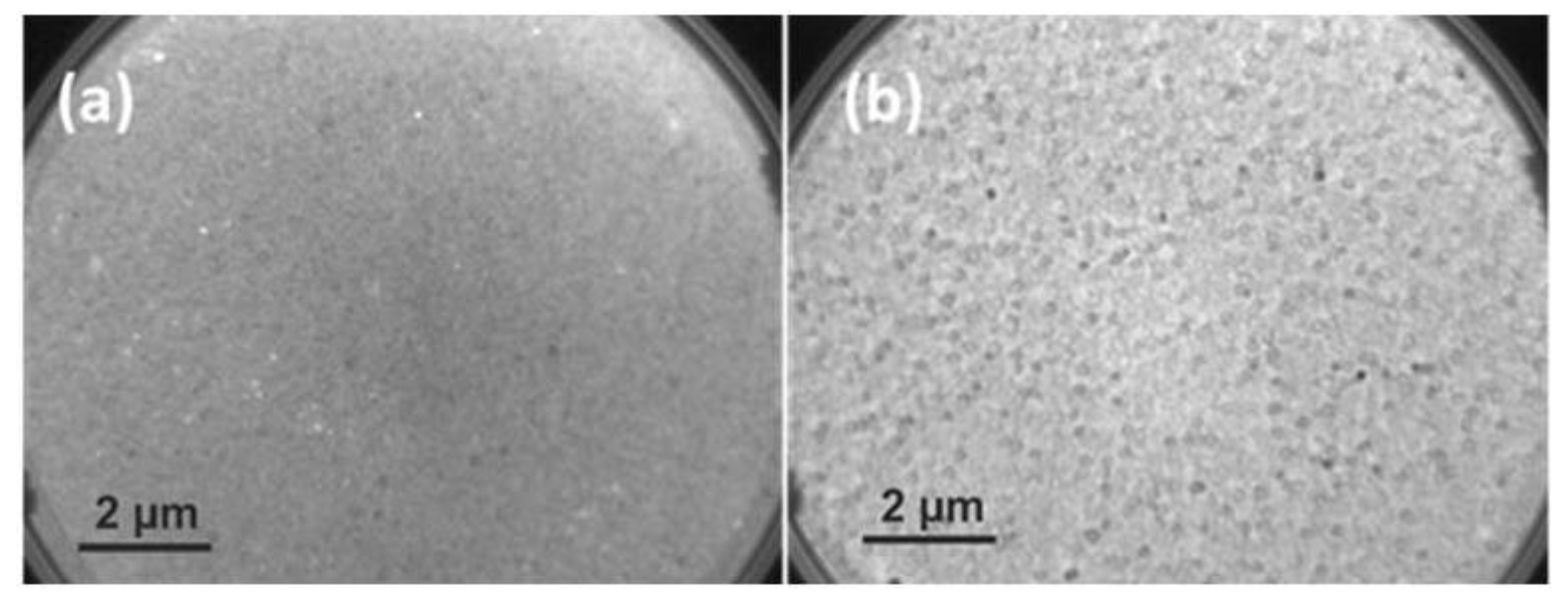
3.1.1. LEEM Analysis
3.1.2. Low-Dose PM4 Synchrotron Analysis of WO3
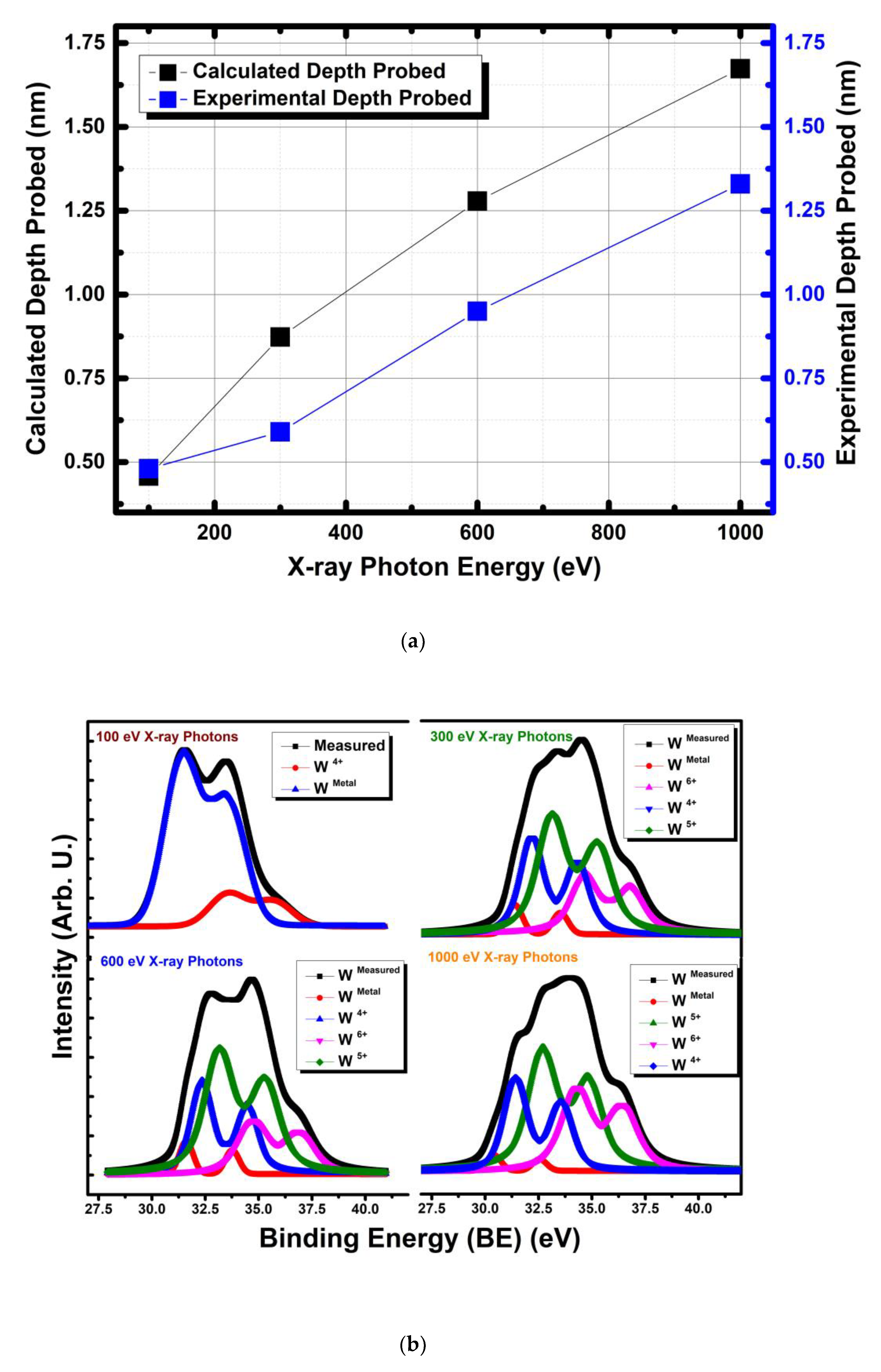
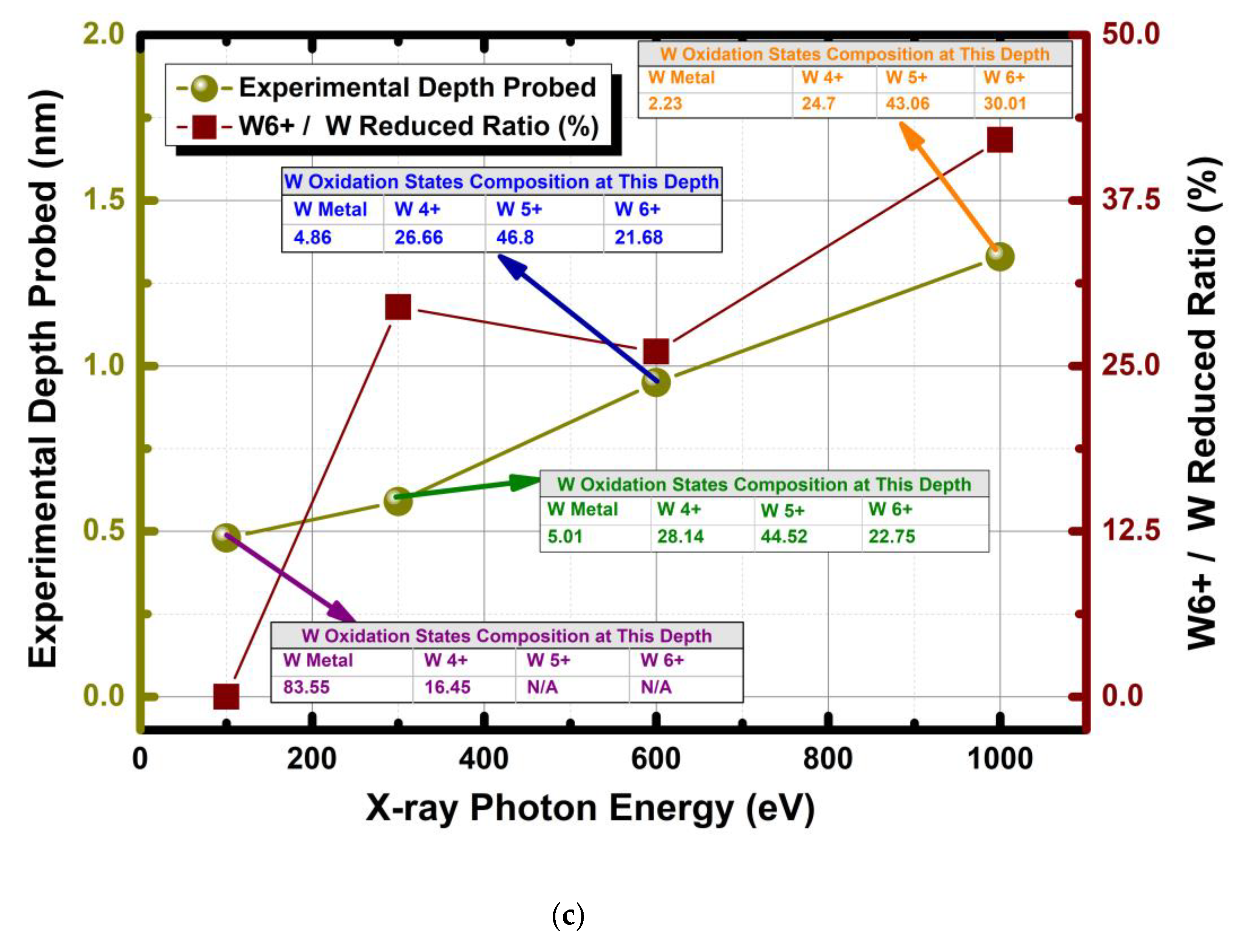
3.1.3. Non Destructive Depth Profiling of WO3 with Synchrotron Based Varying X-ray Photon Energies and Some Remarks on Oxidation State Analysis of WO3
3.2. Van Der Pauw (vdP) Electrical Resistivity Measurements and Chemical Sensor Testing for NO2
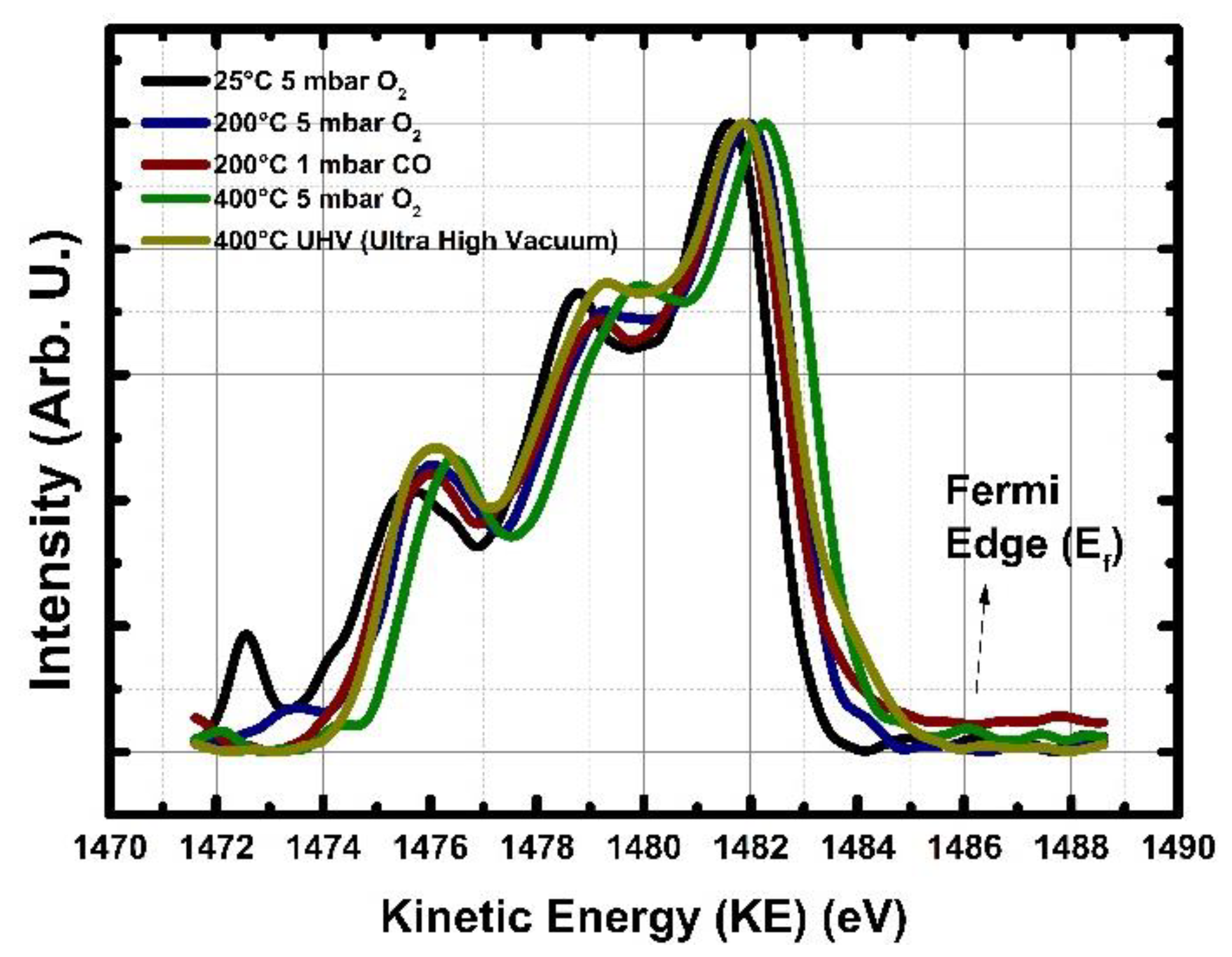
3.3. Near Ambient Pressure (NAP) XPS Analysis of SnO2 and Quantification of Chemisorbed Oxygen Species as a Function of O2 and CO Exposure and Temperature
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wildfire, C.; Çiftyürek, E.; Sabolsky, K.; Sabolsky, E.M. Fabrication and Testing of High-Temperature Nano-Derived Resistive-Type Microsensors for Hydrogen Sensing. J. Electrochem. Soc. 2014, 161, B3094–B3102. [Google Scholar] [CrossRef]
- Ciftyurek, E.; Sabolsky, K.; Edward, S.M. Molybdenum and tungsten oxide based gas sensors for high temperature detection of environmentally hazardous sulfur species. Sens. Actuators B Chem. 2016, 237, 262–274. [Google Scholar] [CrossRef] [Green Version]
- Ciftyurek, E.; Katarzyna, S.; Edward, S.M. High temperature selective sensing of hydrogen with MgO-modified SrMoO4 micro-fibers. Sens. Actuators B Chem. 2017, 249, 296–310. [Google Scholar] [CrossRef] [Green Version]
- Sabolsky, E.; Ciftyurek, E.; Wildfire, C.; Katarzyna, S.; Jonathan, T.; Kostas, S.; Evans, T. Nano-Derived Microsensors for Monitoring Gas Species in Harsh-Environments. ECS Trans. 2014, 61, 375–385. [Google Scholar] [CrossRef]
- Barsan, N.; Klaus, S. Basiscs of Semiconducting Metal Oxide-Based Gas Sensors, Metal Oxides Series: Gas Sensors Based on Conducting Metal Oxides; Elseiver: Amsterdam, The Netherlands, 2018; p. 292. [Google Scholar]
- Brundle, C.R.; Evans, C.A.; Wilson, S., Jr. Encyclopedia of Materials Characterization; Gulf Professional Publishing: Houston, TX, USA, 1992. [Google Scholar]
- NIST Standard Reference Database 71. Available online: https://www.nist.gov/srd/nist-standard-reference-database-71 (accessed on 29 October 2019).
- Hübner, M.; Koziej, D.; Bauer, M.; Barsan, N.; Kvashnina, K.; Rossell, M.D.; Weimar, U.; Grunwaldt, J.D. The Structure and Behavior of Platinum in SnO2-Based Sensors under Working Conditions. Angew. Chem. Int. Ed. 2011, 50, 2841–2844. [Google Scholar] [CrossRef]
- Hirsch, O.; Kvashnina, K.O.; Luo, L.; Süess, M.J.; Glatzel, P.; Koziej, D. High-energy resolution X-ray absorption and emission spectroscopy reveals insight into unique selectivity of La-based nanoparticles for CO2. Proc. Natl. Acad. Sci. USA 2015, 112, 15803–15808. [Google Scholar] [CrossRef] [PubMed]
- Bare, S.R.; Griffiths, K.; Lennard, W.N.; Tang, H.T. Generation of atomic oxygen on Ag(lll) and Ag(ll0) using NO22 a TPD, LEED, HREELS, XPS and NRA study. Surf. Sci. 1995, 342, 185–198. [Google Scholar] [CrossRef]
- Barteau, M.A.; Madix, R.J. A Photelectron Spectroscopic Investigation of The Interaction Between H2O and Oxygen on Ag(110). Surf. Sci. 1984, 140, 108–122. [Google Scholar] [CrossRef]
- Barteau, M.; Ko, E.; Madix, R. The Adsoprtion of CO, O2 and H2 on Pt(100)-(5×20). Surf. Sci. 1981, 102, 99–117. [Google Scholar] [CrossRef]
- Barteau, M.A. Organic Reactions at Well-Defined Oxide Surfaces. Chem. Rev. 1996, 96, 1413–1430. [Google Scholar] [CrossRef]
- Ciftyurek, E.; Wilken, M.; Zanders, D.; Mai, L.; Devi, A.; Schierbaum, K. Monitoring Surface Stoichiometry, Work Function and Valance Band of Tungsten Oxide (WO3), Molybdenum Oxide (MoO3) and Tin Oxide (SnO2) Thin Films as a Function of Temperature and Oxygen Partial Pressure with Advanced Surface Sensitive Techniques for Chemical Sensing Applications. MDPI Proc. 2019, 14, 27. [Google Scholar] [CrossRef]
- Ciftyurek, E. Characterization of Atomic Layer Deposited (ALD) Novel Ultra-Thin Films for Ultra-Sensitive Chemical Sensors. 20177074, CERIC Proposal. 2018. [Google Scholar]
- 5th Annual APXPS Workshop 2018. Available online: https://www.google.com.hk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=2ahUKEwj398XZy8XlAhXbyYsBHV5kCscQFjAAegQIABAC&url=http%3A%2F%2Fwww.fhi-berlin.mpg.de%2Facnew%2Fapxps-2018%2F5th_APXPS_Workshop_Book_of_abstracts.pdf&usg=AOvVaw00Qsqb3xcjyuqDXtl9znNr (accessed on 29 October 2019).
- Tissot, H. Beyond the Gap of Pressure: XPS Studies of Interfaces at Near Ambient Pressures. Ph.D. Thesis, Université Pierre et Marie Curie, Paris, France, 2014. [Google Scholar]
- Azad, M.; Akbar, S.A.; Mhaisalkar, S.G.; Birkefeld, L.D.; Goto, K.S. Solid State Gas Sensors A review. J. Electrochem. Soc. 1992, 139, 3690–3701. [Google Scholar] [CrossRef]
- Kawabe, T.; Tabata, K.; Suzuki, E.; Yamaguchi, Y.; Nagasawa, Y. Electronic States of Chemisorbed Oxygen Species and Their Mutually Related Studies on SnO2 Thin Film. J. Phys. Chem. B 2001, 105, 4239–4244. [Google Scholar] [CrossRef]
- Wildfire, C.; Çiftyürek, E.; Sabolsky, K.; Sabolsky, E.M. Investigation of doped-gadolinium zirconate nanomaterials for high-temperature hydrogen sensor applications. J. Mater. Sci. 2014, 49, 4735–4750. [Google Scholar] [CrossRef]
- Eranna, G.; Joshi, B.C.; Runthala, D.P.; Gupta, R.P. Oxide Materials for Development of Integrated Gas Sensors-A Comprehensive Review. Crit. Rev. Solid State Mater. Sci. 2010, 29, 111–188. [Google Scholar] [CrossRef]
- Choi, U.S.; Sakai, G.; Shimanoe, K.; Yamazoe, N. Sensing properties of Au-loaded SnO2–Co3O4 composites to CO and H2. Sens. Actuators B Chem. 2005, 107, 397–401. [Google Scholar] [CrossRef]
- Egashira, M.; Shimizu, Y.; Takao, Y. Trimethylamine Sensor based on Semiconductive Metal Oxides for Detection of Fish Freshness. Sens. Actuators B Chem. 1990, 1, 108–112. [Google Scholar] [CrossRef]
- Yamazoe, N.; Kurokawa, Y.; Seiyama, T. Effects of additives on semiconductor gas sensors. Sens. Actuators 1983, 174, 283–289. [Google Scholar] [CrossRef]
- Matusko, P.; Yatsimirskii, V.K.; Maksimovich, N.P.; Nikitina, N.V.; Silenko, P.M.; Ruchko, V.P.; Ishchenko, V.B. Sensitivity to hydrogen of sensor materials based on SnO2 promoted with 3d metals. Theor. Exp. Chem. 2008, 442, 128–133. [Google Scholar] [CrossRef]
- Software for Spectroscopy Data Measurement and Processing. 2019. Available online: https://www.kolibrik.net/kolxpd/ (accessed on 30 September 2019).
- McCafferty, E.; Wightman, J. Determination of the Concentration of SurfaceHydroxyl Groups on Metal Oxide Films by aQuantitative XPS Method. Surf. Interface Anal. 1998, 26, 549–564. [Google Scholar] [CrossRef]
- Gaggiotti, G.; Galdikas, A.; Kaciulis, S.; Mattogno, G.; Setkus, A. Surface chemistry of tin oxide based gas sensors. J. Appl. Phys. 1994, 76, 4467. [Google Scholar] [CrossRef]
- Shimizu, Y.; Matsunaga, N.; Hyodo, T.; Egashira, M. Improvement of SO2 sensing properties of WO3 by noble metal loading. Sens. Actuators B Chem. 2016, 77, 35. [Google Scholar] [CrossRef]
- Heo, J.; Hock, A.S.; Gordon, R.G. Low Temperature Atomic Layer Deposition of Tin Oxide. Chem. Mater. 2010, 22, 4964–4973. [Google Scholar] [CrossRef]
- Shanthi, E.; Dutta, V.; Banerjee, A.; Chopra, K.L. Electrical and optical properties of undoped and antimony-doped tin oxide films. J. Appl. Phys. 1980, 51, 6243. [Google Scholar] [CrossRef]
- Senthilkumar, V.; Vickraman, P. Structural, optical and electrical studies on nanocrystalline tin oxide (SnO2) thin films by electron beam evaporation technique. J. Mater. Sci. Mater. Electron. 2010, 21, 578–583. [Google Scholar] [CrossRef]
- Kojima, M.; Kato, H.; Imai, A.; Yoshida, A. Electronic conduction of tin oxide thin films prepared by chemical vapor deposition. J. Appl. Phys. 1988, 64, 1902. [Google Scholar] [CrossRef]
- Banarjee, R.; Das, D. Propoerties of Tin Oxide Films Prepared by Reactive Electron Beam Evaporation. Thin Solid Film. 1987, 149, 291–301. [Google Scholar] [CrossRef]
- Bruno, L.; Pijolat, C.; Lalauze, R. Tin dioxide thin-film gas sensor prepared by chemical vapour deposition: Influence of grain size and thickness on the electrical properties. Sens. Actuators B Chem. 1994, 18, 195–199. [Google Scholar] [CrossRef] [Green Version]
- Marom, H.; Eizenberg, M. The effect of surface roughness on the resistivity increase in nanometric dimensions. J. Appl. Phys. 2006, 99, 123705. [Google Scholar] [CrossRef]





| W6+ 4f7/2 | W5+ 4f7/2 | O in Water/Hydroxide(H2O/OH−) | O in Chemisorbed () | O Lattice (in WO3) | O Lattice Reduced (in WO3−x) | |
|---|---|---|---|---|---|---|
| Binding Energy [eV] | 35.66 | 34.44 | 533.49 | 532.37 | 531.41 | 530.31 |
| Doublet Separation [eV] | 2.15 | 2.17 | NA | NA | NA | NA |
| Full Width Half Maximum Values (FWHM) [eV] | 0.81 (gwid) 0.15 (lwid) | 0.77 (gwid) 0.31 (lwid) | 1.96 | 1.60 | 1.21 | 0.8 |
| Relative amount [at.%.] | 93.88 | 6.12 | 1.52 | 29.73 | 40.58 | 28.17 |
| Natural broadening of X-ray photons [eV] | 0.06 | 0.06 | 0.1 | 0.1 | 0.1 | 0.1 |
| O 1s Spectrum upon Exposure to Below Given, O2, CO Gases at Different Temperatures | O in Water Hydroxides (H2O/OH−) BE (eV) and Concentration [at.%.] | O in Chemisorbed BE (eV) and Concentration [at.%.] | O lattice (in SnO2), BE (eV) and Concentration [at.%.] | O lattice Reduced (in SnO2), BE (eV) and Concentration [at.%.] |
|---|---|---|---|---|
| 25 °C UHV (Ultra High Vacuum) | 532.4/5.4 | 531.9/15.8 | 531.0/76 | 530.4/2.8 |
| 25 °C 5 mbar O2 | 532.8/2.5 | 532.0/16.8 | 530.9/77.9 | 529.5/2.8 |
| 200 °C 5 mbar O2 | 532.4/6.4 | 531.8/13.3 | 530.9/77.7 | 529.8/2.6 |
| 200 °C 5 mbar O2 to 1 mbar CO | 532.5/8.0 | 531.7/6.4 | 530.9/81 | 529.7/4.6 |
| 200 °CK 1 mbar CO | 532.7/6.7 | 531.9/3.1 | 531.0/79 | 530.1/11.2 |
| 400 °C 5 mbar O2 | 532.7/2.5 | 531.7/22.1 | 530.9/71.1 | 529.8/4.3 |
| 400 °C UHV (Ultra High Vacuum) | NA/NA | 532.5/3.2 | 531.1/52.3 | 530.9 and 529.7/44.5 (including both Sn2+ and SnX, 2 < x < 3 phases both shown to be yellow in the Figure 6a) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciftyürek, E.; Šmíd, B.; Li, Z.; Matolín, V.; Schierbaum, K. Spectroscopic Understanding of SnO2 and WO3 Metal Oxide Surfaces with Advanced Synchrotron Based; XPS-UPS and Near Ambient Pressure (NAP) XPS Surface Sensitive Techniques for Gas Sensor Applications under Operational Conditions. Sensors 2019, 19, 4737. https://doi.org/10.3390/s19214737
Ciftyürek E, Šmíd B, Li Z, Matolín V, Schierbaum K. Spectroscopic Understanding of SnO2 and WO3 Metal Oxide Surfaces with Advanced Synchrotron Based; XPS-UPS and Near Ambient Pressure (NAP) XPS Surface Sensitive Techniques for Gas Sensor Applications under Operational Conditions. Sensors. 2019; 19(21):4737. https://doi.org/10.3390/s19214737
Chicago/Turabian StyleCiftyürek, Engin, Břetislav Šmíd, Zheshen Li, Vladimír Matolín, and Klaus Schierbaum. 2019. "Spectroscopic Understanding of SnO2 and WO3 Metal Oxide Surfaces with Advanced Synchrotron Based; XPS-UPS and Near Ambient Pressure (NAP) XPS Surface Sensitive Techniques for Gas Sensor Applications under Operational Conditions" Sensors 19, no. 21: 4737. https://doi.org/10.3390/s19214737





