Application of Raman Spectroscopy to Working Gas Sensors: From in situ to operando Studies
Abstract
:1. Introduction
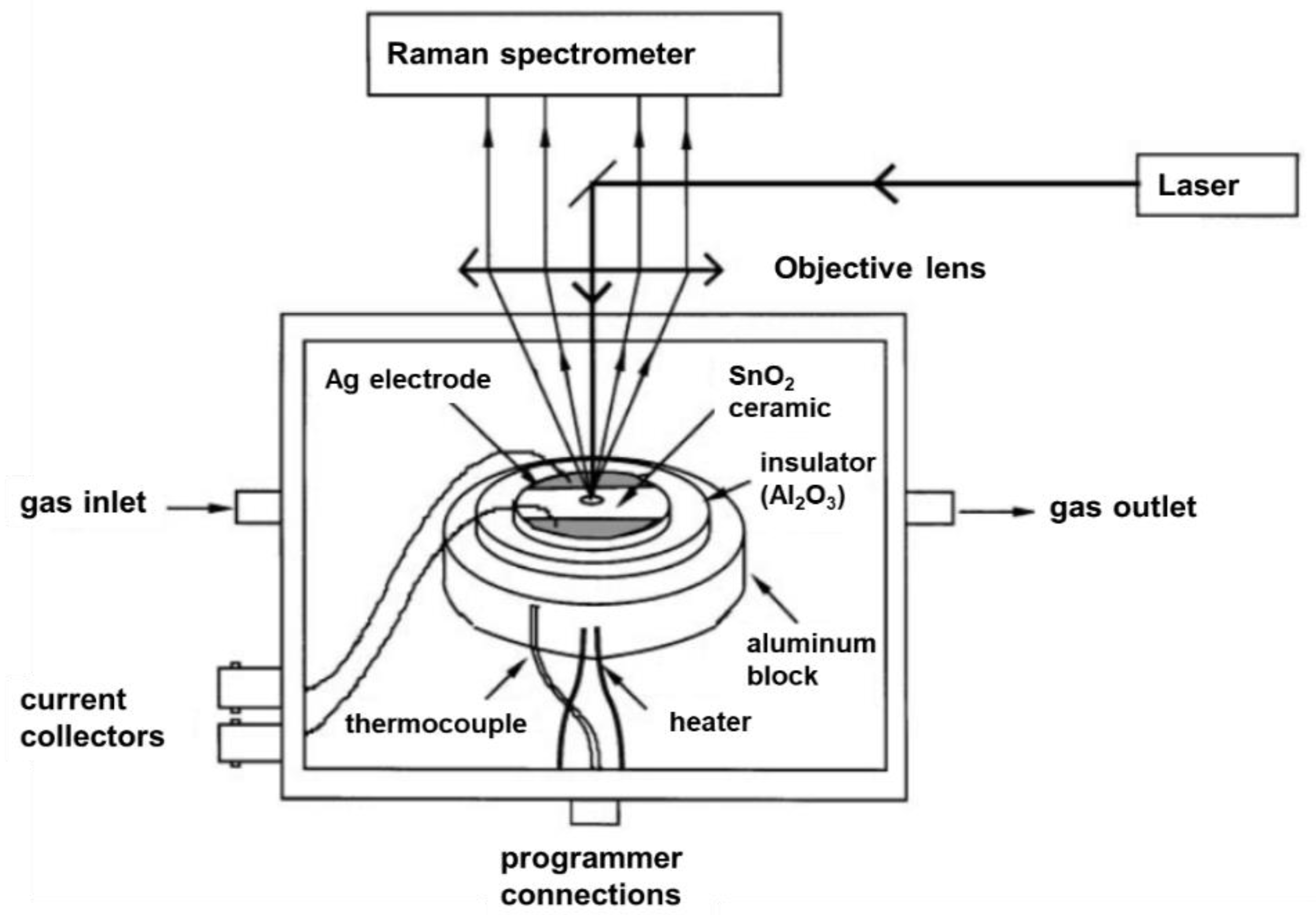
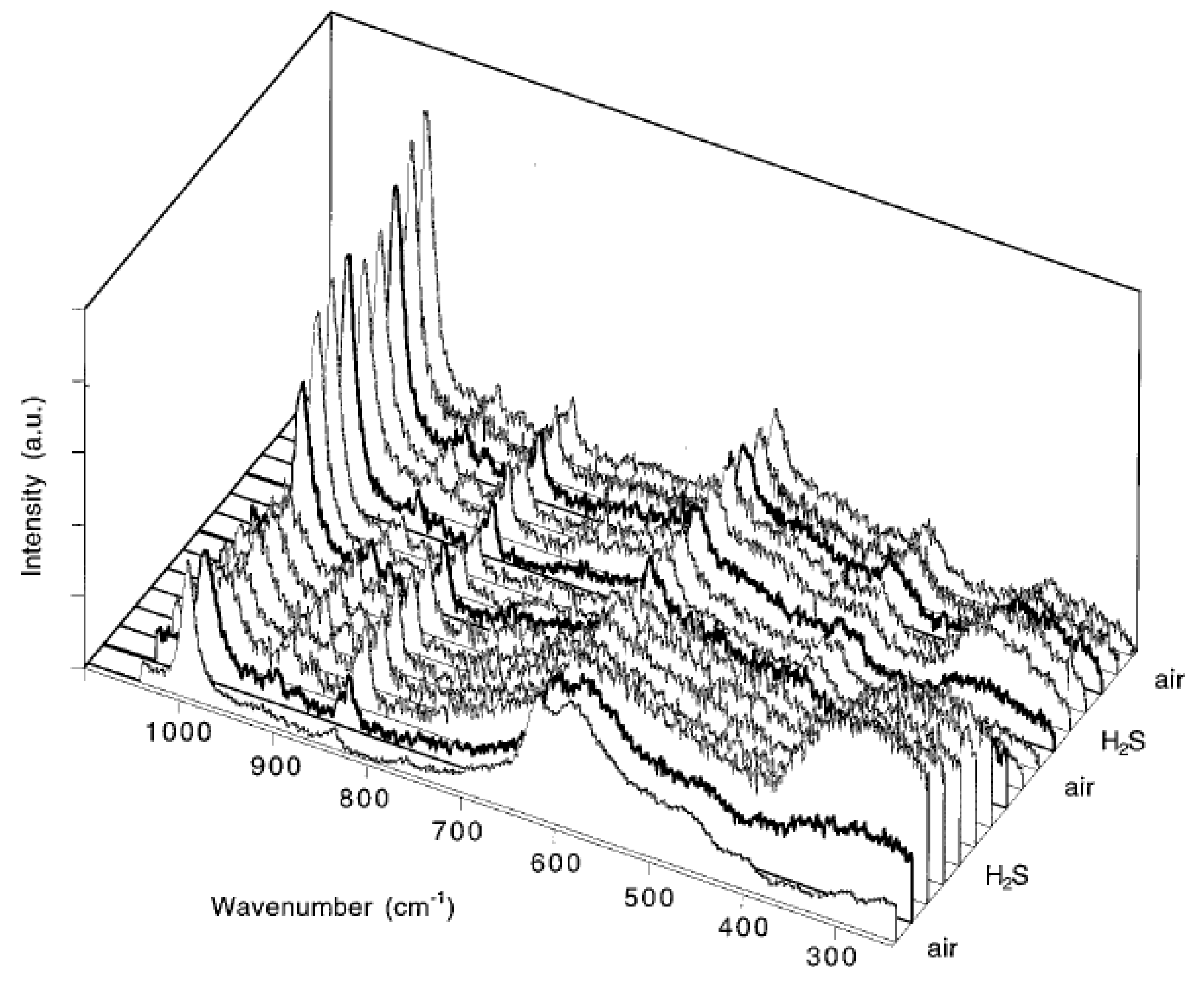
2. Raman Cells for In situ und Operando Spectroscopy on Gas Sensors
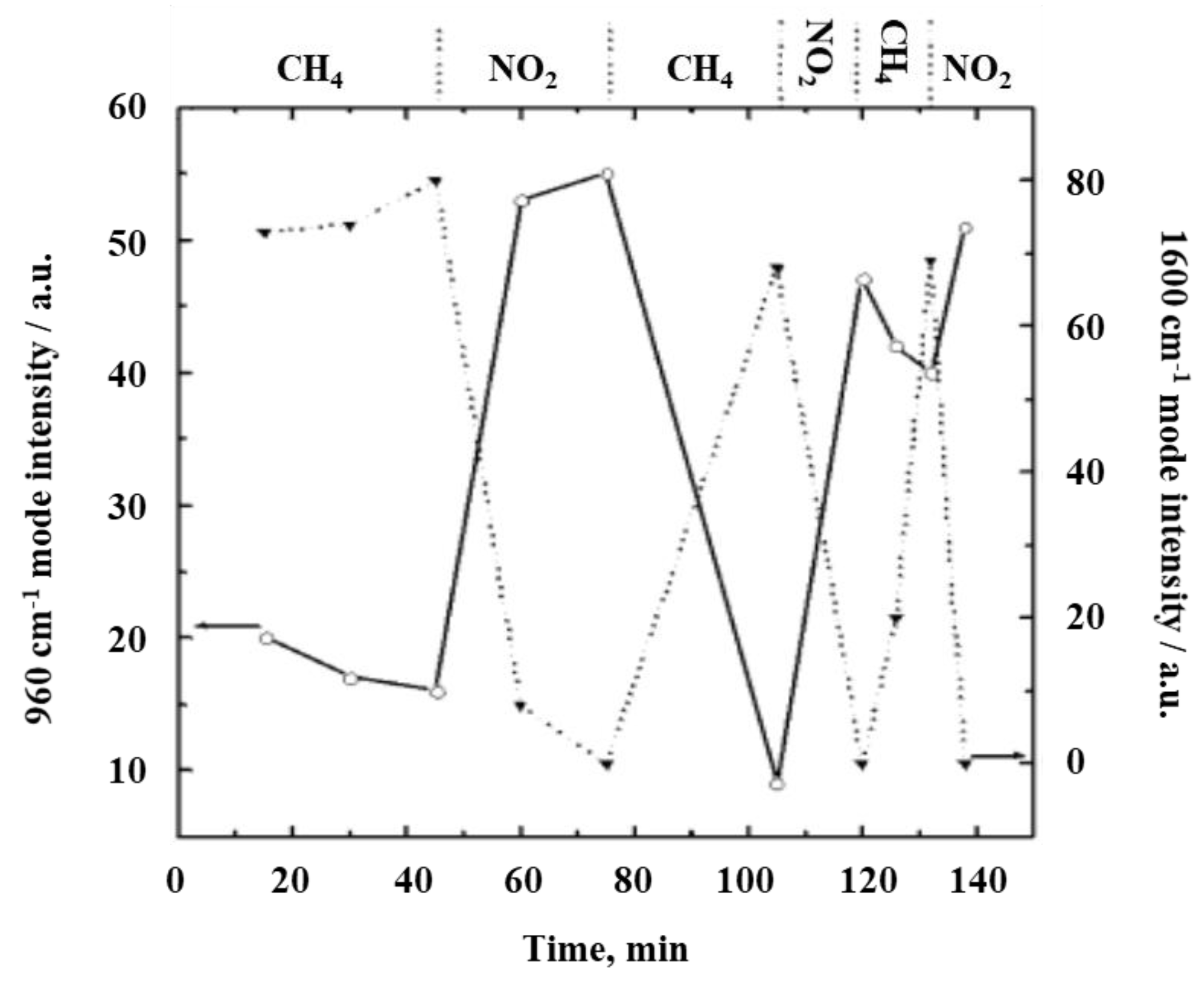
3. Towards Operando Raman Spectroscopy on Gas Sensors
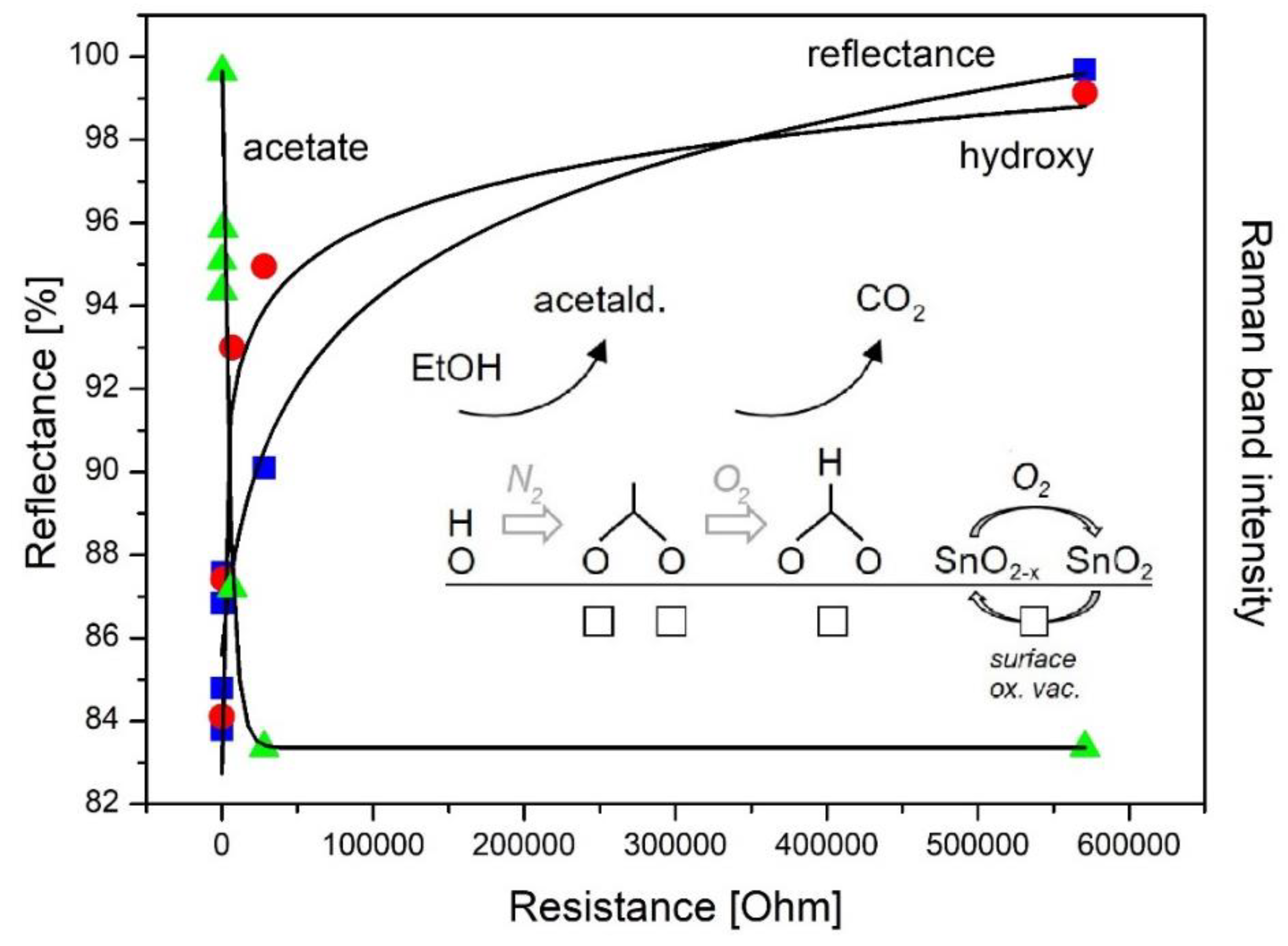
4. Application of Operando Raman Spectroscopy to Gas Sensors
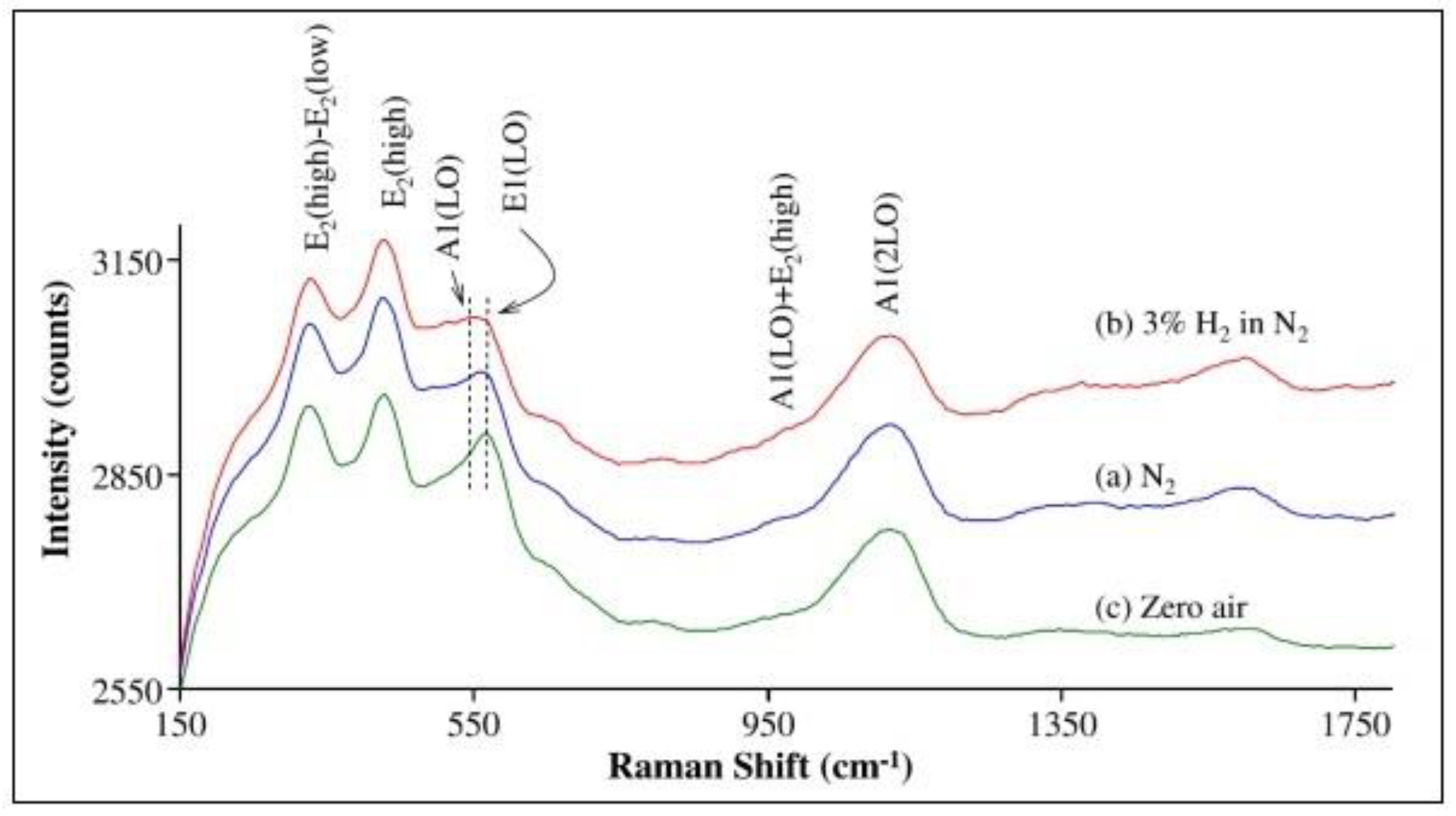
4.1. Operando Raman Spectroscopy
4.2. Operando Surface-Enhanced Raman Spectroscopy (SERS)
4.3. Multiple Operando Spectroscopy
5. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Williams, D.E. Semiconducting Oxides as Gas-Sensitive Resistors. Sens. Actuators B Chem. 1999, 57, 1–16. [Google Scholar] [CrossRef]
- Korotcenkov, G. Metal Oxides for Solid-State Gas Sensors: What Determines our Choice? Mater. Sci. Eng. B 2007, 139, 1–23. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor Metal Oxide Gas Sensors: A Review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Gurlo, A.; Riedel, R. In Situ and Operando Spectroscopy for Assessing Mechanisms of Gas Sensing. Angew. Chem. Int. Ed. 2007, 46, 3826–3848. [Google Scholar] [CrossRef] [PubMed]
- Sänze, S.; Gurlo, A.; Hess, C. Monitoring Gas Sensors at Work: Operando Raman-FTIR Study of Ethanol Detection by Indium Oxide. Angew. Chem. Int. Ed. 2013, 52, 3607–3610. [Google Scholar] [CrossRef] [PubMed]
- Elger, A.-K.; Hess, C. Elucidating the Mechanism of Working SnO2 Gas Sensors Using Combined Operando UV/Vis, Raman, and IR Spectroscopy. Angew. Chem. Int. Ed. 2019, 58, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Degler, D.; Wicker, S.; Weimar, U.; Barsan, N. Identifying the Active Oxygen Species in SnO2 Based Gas Sensing Materials: An Operando IR Spectroscopy Study. J. Phys. Chem. C 2015, 119, 11792–11799. [Google Scholar] [CrossRef]
- Koziej, D.; Hübner, M.; Barsan, N.; Weimar, U.; Sikora, M.; Grunwaldt, J.-D. Operando X-Ray Absorption Spectroscopy Studies on Pd-SnO2 Based Sensors. Phys. Chem. Chem. Phys. 2009, 11, 8620–8625. [Google Scholar] [CrossRef]
- Degler, D.; Barz, N.; Dettinger, U.; Peisert, H.; Chasse, T.; Weimar, U.; Barsan, N. Extending the Toolbox for Gas Sensor Research: Operando UV/vis Diffuse Reflectance Spectroscopy on SnO2-based Gas Sensors. Sens. Actuators B Chem. 2016, 224, 256–259. [Google Scholar] [CrossRef]
- Hübner, M.; Koziej, D.; Bauer, M.; Barsan, N.; Kvashnina, K.M.; Rossell, D.; Weimar, U.; Grunwaldt, J.-D. The Structure and Behavior of Platinum in SnO2-Based Sensors under Working Conditions. Angew. Chem. Int. Ed. 2011, 50, 2841–2844. [Google Scholar] [CrossRef]
- Wicker, S.; Guiltat, M.; Weimar, U.; Hémeryck, A.; Barsan, N. Ambient Humidity Influence on CO Detection With SnO2 Gas Sensing Materials. A Combined DRIFTS/DFT Investigation. J. Phys. Chem. C 2017, 121, 25064–25073. [Google Scholar] [CrossRef]
- Gurlo, A.; Riedel, R. Active Metal Electrode-Oxide Interface in Gas Sensor Operation Probed by In Situ and Time-Resolved X-Ray Spectroscopy. ChemPhysChem 2010, 11, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Rumyantseva, M.N.; Gaskov, A.M.; Rosman, N.; Pagnier, T.; Morante, J.R. Raman Surface Vibration Modes in Nanocrystalline SnO2: Correlation With Gas Sensor Performances. Chem. Mater. 2005, 17, 893–901. [Google Scholar] [CrossRef]
- Mihaila, M. Correlations Phonon Spectrum-Sensitivity in Metal-Oxide Gas Sensors. Procedia Eng. 2014, 87, 1609–1612. [Google Scholar] [CrossRef]
- Du, J.; Huang, L.; Chen, Z.; Kang, D.J. A Controlled Method to Synthesize Hydrid In2O3/Ag Nanochains and Nanoparticles: Surface-Enhanced Raman Scattering. J. Phys. Chem. C 2009, 113, 9998–10004. [Google Scholar] [CrossRef]
- Kappler, J.; Bârsan, N.; Weimar, U.; Dièguez, A.; Alay, J.L.; Romano-Rodriguez, A.; Morante, J.R.; Göpel, W. Correlation Between XPS, Raman and TEM Measurements and the Gas Sensitivity of Pt and Pd Doped SnO2 Based Gas Sensors. Fresenius J. Anal. Chem. 1998, 361, 110–114. [Google Scholar] [CrossRef]
- Manjula, P.; Satyanarayana, L.; Swarnalatha, Y.; Manorama, S.V. Raman and MASNMR Studies to Support the Mechanism of Low Temperature Hydrogen Sensing by Pd Doped Mesoporous SnO2. Sens. Actuators B Chem. 2009, 138, 28–34. [Google Scholar] [CrossRef]
- Mohanapriya, P.; Segawa, H.; Watanabe, K.; Watanabe, K.; Samitsu, S.; Natarajan, T.S.; Jaya, N.V.; Ohashi, N. Enhanced Ethanol-Gas Sensing Performance of Ce-Doped SnO2 Hollow Nanofibers Prepared by Electrospinning. Sens. Actuators B Chem. 2013, 188, 872–878. [Google Scholar] [CrossRef]
- Wie, X.; Liu, X.; Xub, S. Optical Investigation of the Size Effects on NO2 Adsorption in SnO2 Nanoparticles. Solid State Commun. 2008, 148, 491–495. [Google Scholar]
- Sahu, B.K.; Das, A.; Prasad, A.K.; Mangamma, G. The Role of In-Plane Oxygen Vacancy Defects in SnO2 Nanoparticles for CH4 Sensing. J. Nanosci. Nanotechnol. 2019, 19, 7764–7770. [Google Scholar] [CrossRef]
- Pagnier, T.; Boulova, M.; Sergent, N.; Bouvier, P.; Lucazeau, G. Nanopowders and Nanostructured Oxides: Phase Transitions and Surface Reactivity. J. Raman Spectrosc. 2007, 38, 756–761. [Google Scholar] [CrossRef]
- Pagnier, T.; Boulova, M.; Galerie, A.; Gaskov, A.; Lucazeau, G. In situ Coupled Raman and Impedance Measurements of the Reactivity of Nanocrystalline SnO2 versus H2S. J. Solid State Chem. 1999, 143, 86–94. [Google Scholar] [CrossRef]
- Pagnier, T.; Boulova, M.; Galerie, A.; Gaskov, A.; Lucazeau, G. Reactivity of SnO2-CuO Nanocrystalline Materials with H2S: A Coupled Electrical and Raman Spectroscopic Study. Sens. Actuators B Chem. 2000, 71, 134–139. [Google Scholar] [CrossRef]
- Boulova, M.; Gaskov, A.; Lucazeau, G. Tungsten Oxide Reactivity Versus CH4, CO and NO2 Molecules Studied by Raman Spectroscopy. Sens. Actuators B Chem. 2001, 81, 99–106. [Google Scholar] [CrossRef]
- Sergent, N.; Epifani, M.; Pagnier, T. In situ Raman Spectroscopy Study of NO2 Adsorption onto Nanocrystalline Tin(IV) Oxide. J. Raman Spectrosc. 2006, 37, 1272–1277. [Google Scholar] [CrossRef]
- Garcia-Sanchez, R.F.; Ahmido, T.; Casimir, D.; Baliga, S.; Misra, P. Thermal Effects Associated With the Raman Spectroscopy of WO3 Gas-Sensor Material. J. Phys. Chem. A 2013, 117, 13825–13831. [Google Scholar] [CrossRef] [PubMed]
- Akande, A.A.; Machatine, A.G.J.; Masina, B.; Chimowa, G.; Matsoso, B.; Roro, K.; Duvenhage, M.-M.; Swart, H.; Bandyopadhyay, J.; Ray, S.S.; et al. Blue- and Red-Shifts of V2O5 Phonons in NH3 Environment by In Situ Raman Spectroscopy. J. Phys. D Appl. Phys. 2018, 51, 015106. [Google Scholar] [CrossRef]
- Loridant, S.; Abello, L.; Siebert, E.; Lucazeau, G. Correlations Between Structural and Electrical Properties of BaCeO3 Studied by Coupled In-Situ Raman Scattering and Impedance Spectroscopy. Solid State Ionics 1995, 78, 249–258. [Google Scholar] [CrossRef]
- Ou, J.Z.; Yaacon, M.H.; Breedon, M.; Zheng, H.D.; Campbell, J.L.; Latham, K.; Plessis, J.D.; Wlodarski, W.; Kalantar-Zadeh, K. In Situ Raman spectroscopy of H2 Interaction with WO3 Films. Phys. Chem. Chem. Phys. 2011, 13, 7330–7339. [Google Scholar] [CrossRef]
- Wong, K.W.J.; Field, M.R.; Ou, J.Z.; Latham, K.; Spencer, M.J.S.; Yarovsky, I.; Kalantar-zadeh, K. Interaction of Hydrogen With ZnO Nanopowders—Evidence of Hydroxyl Group Formation. Nanotechnology 2012, 23, 015705. [Google Scholar] [CrossRef]
- Sergent, N.; Epifani, M.; Comini, E.; Faglia, G.; Pagnier, T. Interaction of Nanocrystalline Tin Oxide Powder with NO2: A Raman Spectroscopic Study. Sens. Actuators B Chem. 2007, 126, 1–5. [Google Scholar] [CrossRef]
- Xu, K.; Tian, S.; Zhu, J.; Shi, J.; Yu, T.; Yuan, C. High Selectivity of Sulfur-Doped SnO2 in NO2 Detection at Lower Operating Temperatures. Nanoscale 2018, 10, 20761–20771. [Google Scholar] [CrossRef] [PubMed]
- Sänze, S.; Hess, C. Operando Spectroscopic Study of the EtOH Gas Sensing Mechanism of In2O3. IMCS Proc. 2012, 613–615. [Google Scholar] [CrossRef]
- Sänze, S.; Hess, C. Ethanol Gas Sensing by Indium Oxide: An Operando Spectroscopic Raman-FTIR Study. J. Phys. Chem. C 2014, 118, 25603–25613. [Google Scholar] [CrossRef]
- Berka, S.; Fleischer, V.; Hess, C. Shining Light on Indium Oxide Gas Sensors at Work: A Combined Operando Raman/UV-Vis/FT-IR Spectroscopic Study. MDPI Proc. 2019, 14, 11. [Google Scholar] [CrossRef] [Green Version]
- Elger, A.-K.; Baranyai, J.; Hofmann, K.; Hess, C. Direct Operando Spectroscopic Observation of Oxygen Vacancies in Working Ceria-Based Gas Sensors. ACS Sens. 2019, 4, 1497–1501. [Google Scholar] [CrossRef]
- Filtschew, A.; Hess, C. Unravelling the Mechanism of NO and NO2 storage in Ceria: The Role of Defects and Ce-O Surface Sites. Appl. Catal. B 2018, 237, 1066–1081. [Google Scholar] [CrossRef]
- Yin, W.; Su, J.; Cao, M.; Ni, C.; Cloutier, S.G.; Huang, Z.; Ma, X.; Ren, L.; Hu, C.; Wei, B. In(OH)3 and In2O3 Micro/Nanostructures: Controllable NaOAc-Assisted Microemulsion Synthesis and Raman Properties. J. Phys. Chem. C 2009, 113, 19493–19499. [Google Scholar] [CrossRef]
- Bielz, T.; Lorenz, H.; Jochum, W.; Kaindl, R.; Klauser, F.; Klötzer, B.; Penner, S. Hydrogen on In2O3: Reducibility, Bonding, Defect Formation, and Reactivity. J. Phys. Chem. C 2010, 114, 9022–9029. [Google Scholar] [CrossRef]
- Yang, R.D.; Tripathy, S.; Li, Y.; Sue, H. Photoluminescence and Micro-Raman Scattering in ZnO Nanoparticles: The Influence of Acetate Adsorption. Chem. Phys. Lett. 2005, 411, 150–154. [Google Scholar] [CrossRef]
- Schrödle, S.; Moore, F.G.; Richmond, G.L. In Situ Investigation of Carboxylate Adsorption at the Fluorite/Water Interface by Sum Frequency Spectroscopy. J. Phys. Chem. C 2007, 111, 8050–8059. [Google Scholar] [CrossRef]
- Schilling, C.; Hofmann, A.; Hess, C.; Ganduglia-Pirovano, M.V. Raman Spectra of Polycrystalline CeO2: A Density Functional Theory Study. J. Phys. Chem. C 2017, 121, 20834–20849. [Google Scholar] [CrossRef]
- Schilling, C.; Hess, C. Real-Time Observation of the Defect Dynamics in Working Au/CeO2 Catalysts by Combined Operando Raman/UV-Vis Spectroscopy. J. Phys. Chem. C 2018, 122, 2909–2917. [Google Scholar] [CrossRef]
- Kneipp, K.; Kneipp, H.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Ultrasensitive Chemical Analysis by Raman Spectroscopy. Chem. Rev. 1999, 99, 2957–2976. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Zhang, L.; Wang, T.; Wan, Q. High Surface-Enhanced Raman Scattering Activity from Au-Decorated Individual and Branched Tin Oxide Nanowires. J. Appl. Phys. 2009, 106, 104316. [Google Scholar] [CrossRef]
- Hu, J.-W.; Zhang, Y.; Li, J.-F.; Liu, Z.; Ren, B.; Sun, S.-G.; Tian, Z.-Q.; Lian, T. Synthesis of Au@Pd Core-Shell Nanoparticles With Controllable Size and Their Application in Surface-Enhanced Raman Spectroscopy. Chem. Phys. Lett. 2005, 4, 354–359. [Google Scholar] [CrossRef]
- Popescu, D.A.; Herrmann, J.-M.; Ensuque, A.; Bozon-Verduraz, F. Nanosized Tin Dioxide: Spectroscopic (UV–VIS, NIR, EPR) and Electrical Conductivity Studies. Phys. Chem. Chem. Phys. 2001, 3, 2522–2530. [Google Scholar] [CrossRef]
- Kim, H.; Kosuda, K.M.; Van Duyne, R.P.; Stair, P.C. Resonance Raman and Surface- and Tip-Enhanced Raman Spectroscopy Methods to Study Solid Catalysts and Heterogeneous Catalytic Reactions. Chem. Soc. Rev. 2010, 39, 4820–4844. [Google Scholar] [CrossRef]
- Waleska, P.; Rupp, S.; Hess, C. Operando Multi-Wavelength and Time-Resolved Raman Spectroscopy: Structural Dynamics of a Supported Vanadia Catalyst at Work. J. Phys. Chem. C 2018, 122, 3386–3400. [Google Scholar] [CrossRef]



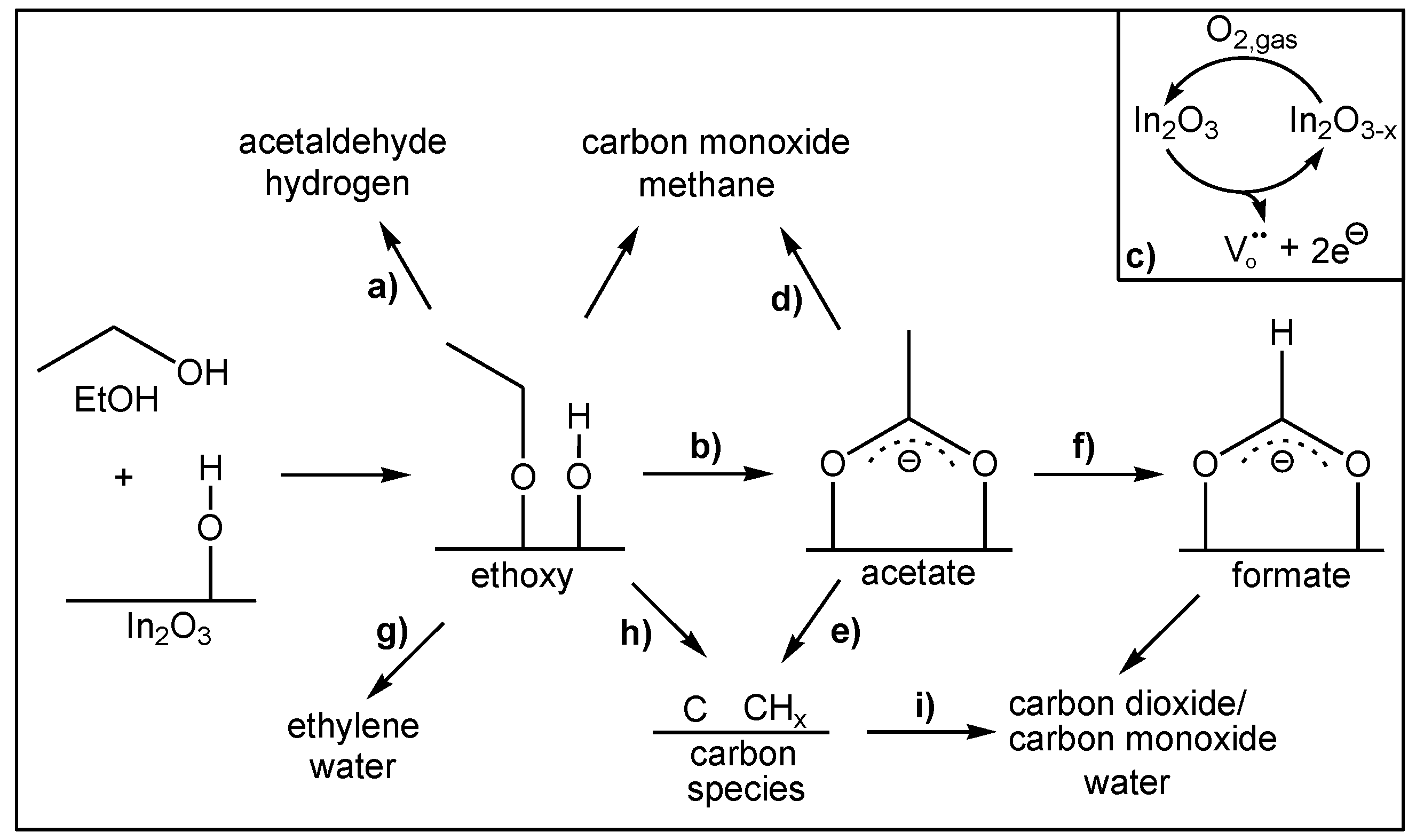
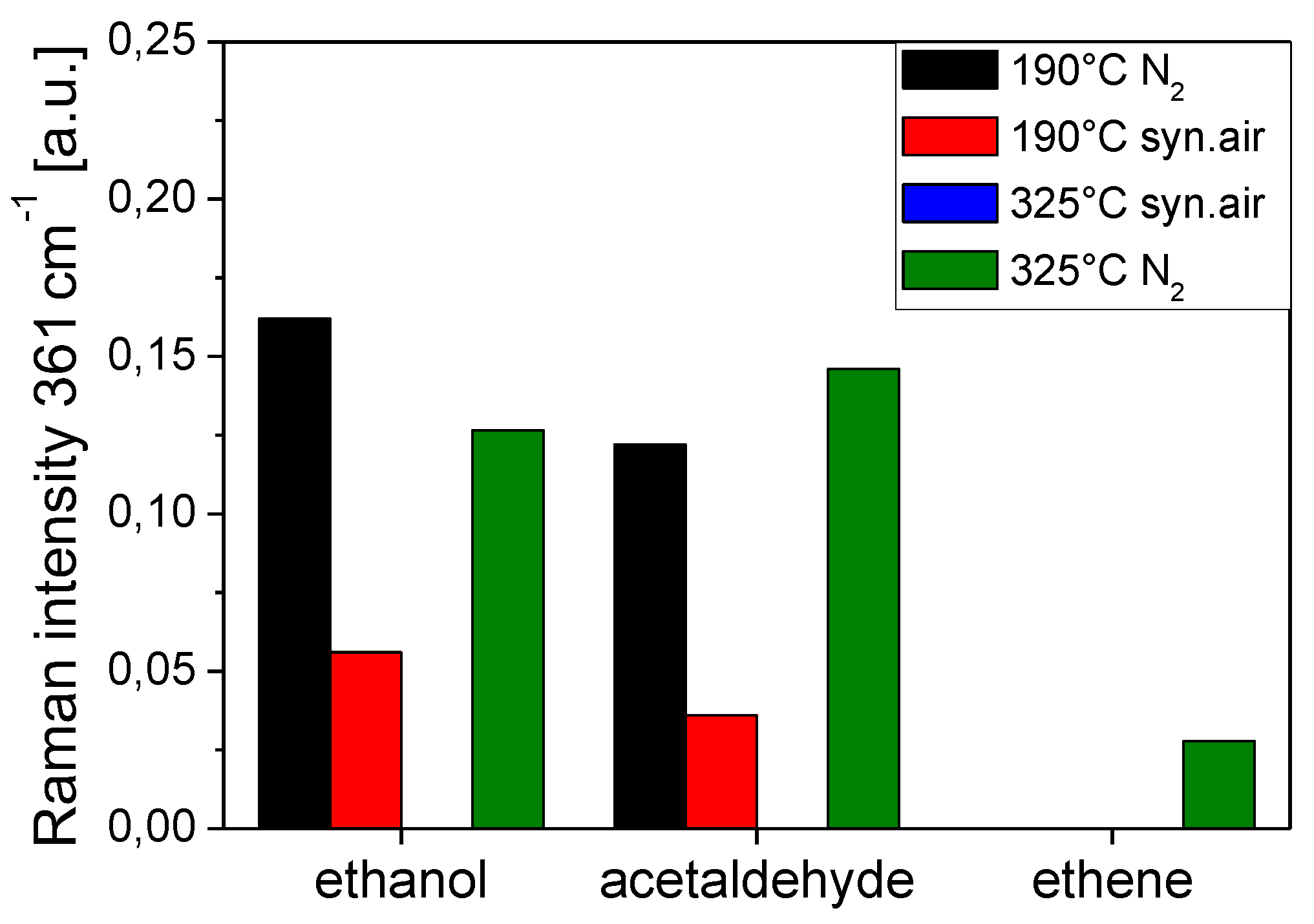
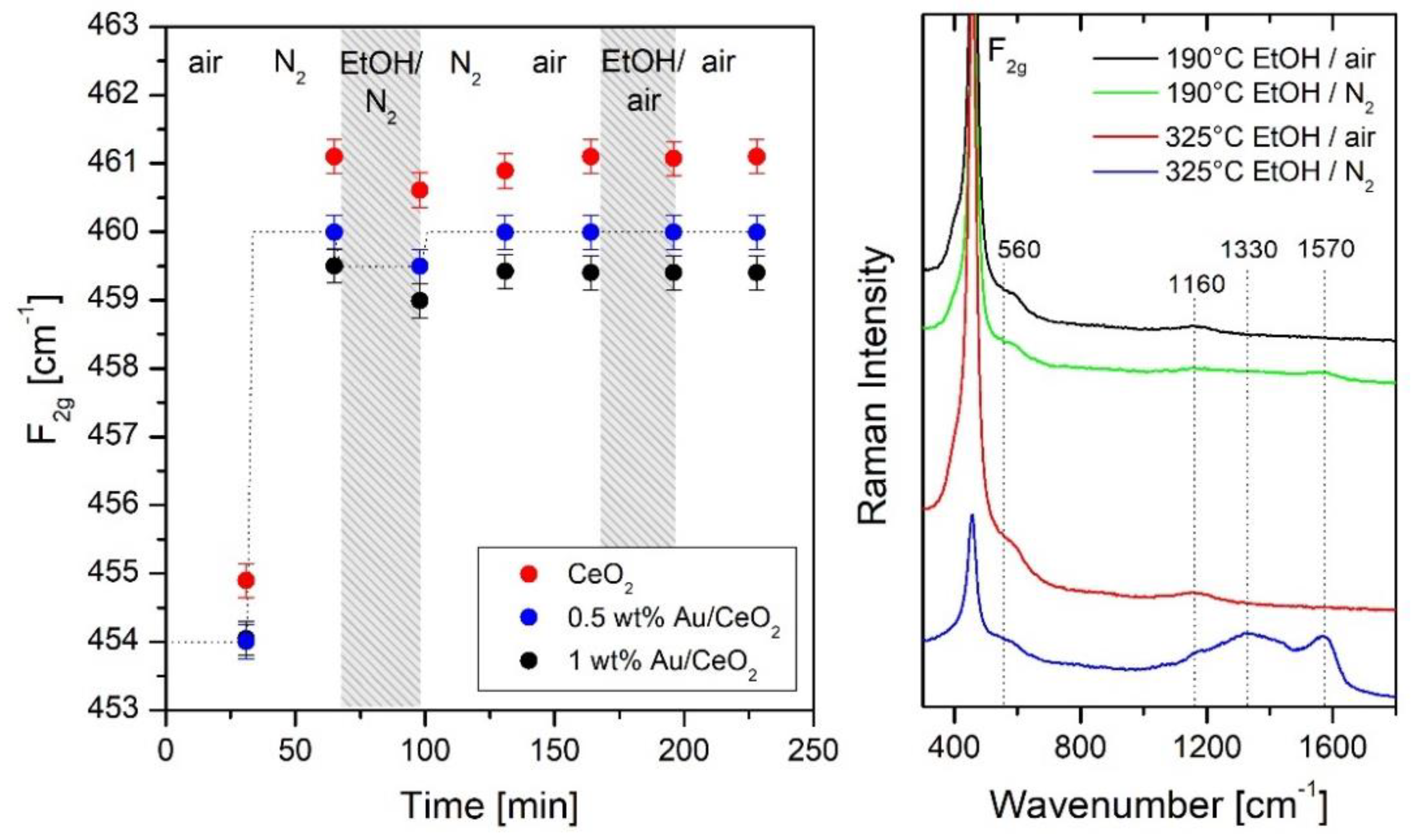
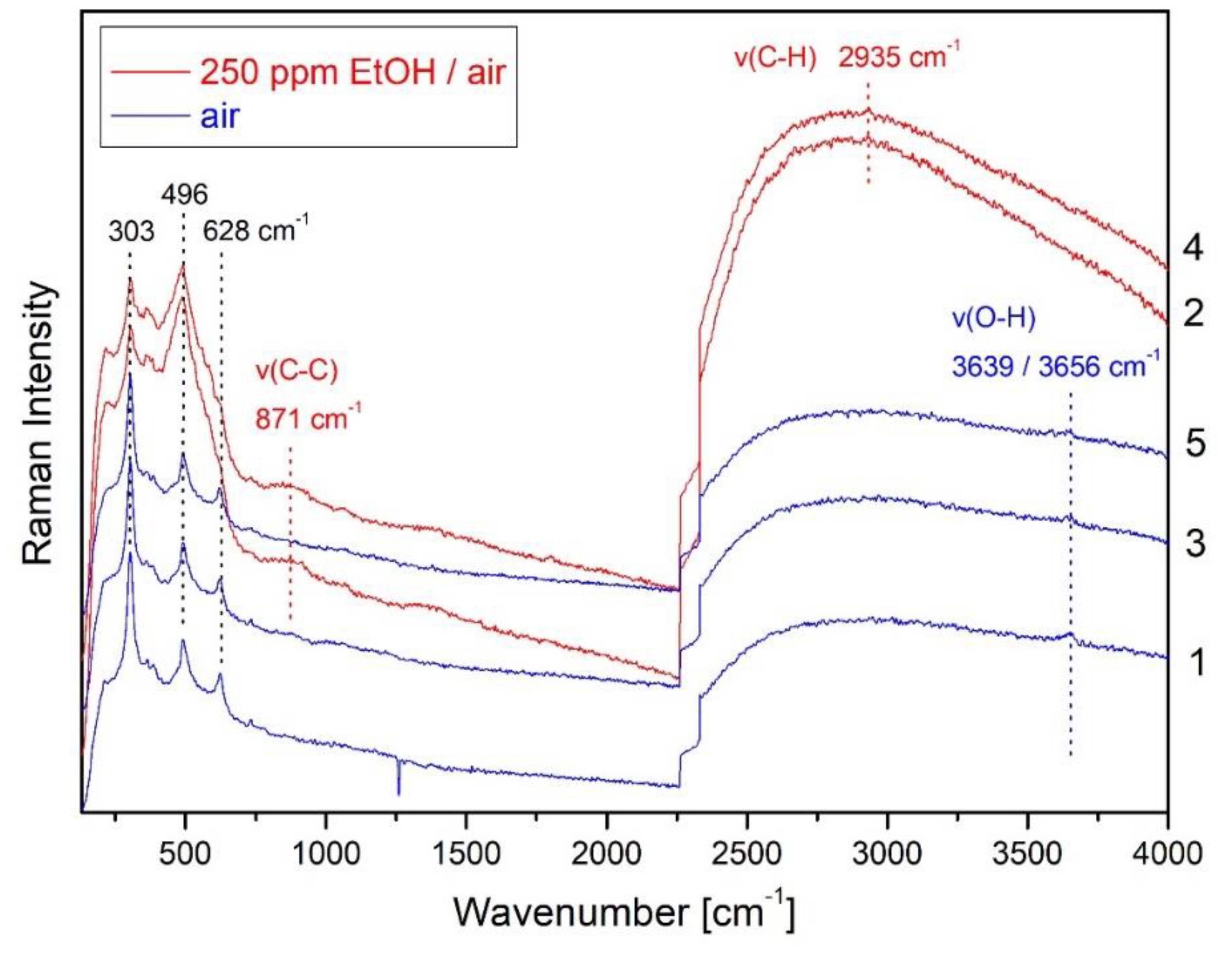

| Metal Oxide | Target Gas | |
|---|---|---|
| In situ | SnO2 | H2S [22] |
| NO2 [25,31] | ||
| CuO/SnO2 | H2S [23] | |
| S/SnO2 | NO2 [32] | |
| WO3 | CH4, CO, NO2 [24] | |
| H2 [29] | ||
| ZnO | H2 [30] | |
| BaCeO3 | Air, N2, CO2 [28] | |
| V2O5 | NH3 [27] | |
| TiO2 | NO2 [31] | |
| Operando | SnO2 | EtOH [6] |
| In2O3 | EtOH, acetald., ethene, CO [5,34,35] | |
| Ag/In2O3 | EtOH, CO [35] | |
| CeO2 | EtOH [36] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elger, A.-K.; Hess, C. Application of Raman Spectroscopy to Working Gas Sensors: From in situ to operando Studies. Sensors 2019, 19, 5075. https://doi.org/10.3390/s19235075
Elger A-K, Hess C. Application of Raman Spectroscopy to Working Gas Sensors: From in situ to operando Studies. Sensors. 2019; 19(23):5075. https://doi.org/10.3390/s19235075
Chicago/Turabian StyleElger, Ann-Kathrin, and Christian Hess. 2019. "Application of Raman Spectroscopy to Working Gas Sensors: From in situ to operando Studies" Sensors 19, no. 23: 5075. https://doi.org/10.3390/s19235075




